


This article presents a comprehensive step-by-step guide to mastering the FDA approval process, detailing essential stages such as:
It underscores the significance of thorough documentation and effective communication with the FDA. Furthermore, it highlights recent advancements in the approval process that can expedite timelines, thereby equipping innovators with the necessary strategies to navigate regulatory challenges successfully.
Navigating the FDA approval process resembles traversing a complex maze, where each turn presents new challenges and opportunities. This step-by-step guide demystifies the intricate journey from initial testing to market entry, highlighting the critical stages that ensure the safety and efficacy of new drugs and medical devices. As innovators strive to bring groundbreaking therapies to patients, understanding the nuances of this process becomes essential—especially in light of recent advancements that promise to expedite approvals.
What strategies can companies employ to not only survive but thrive in this ever-evolving landscape of regulatory requirements?
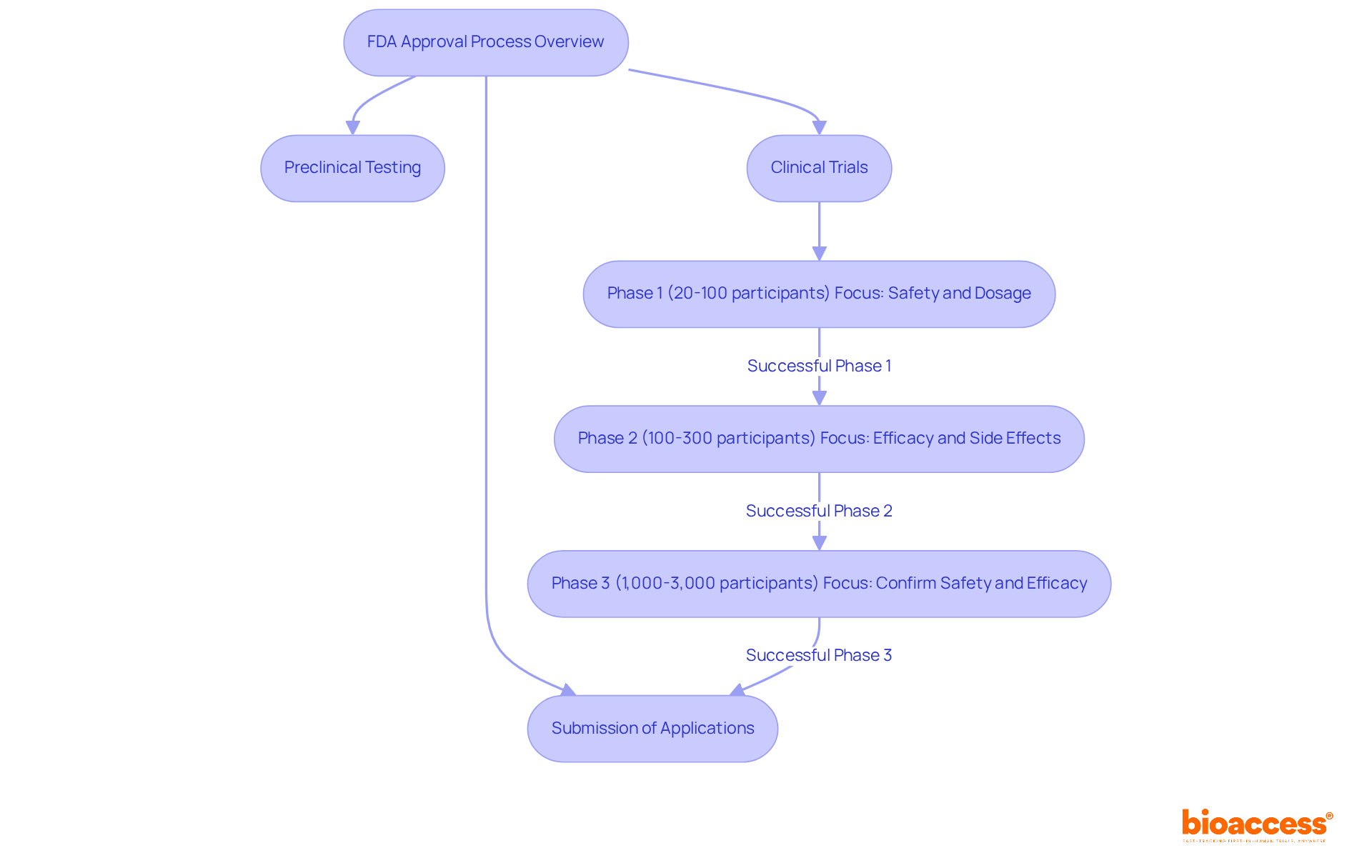
The FDA approval process is an intricate, multi-step journey aimed at guaranteeing the safety and effectiveness of new medications and medical instruments prior to their market entry. This procedure encompasses several essential stages: preclinical testing, clinical trials, and the filing of important applications such as the Investigational New Drug (IND) application and the New Drug Application (NDA). Each phase has distinct requirements and timelines that can vary significantly depending on the product type.
Preclinical Testing: This initial phase involves laboratory and animal studies to gather data on a drug's safety and biological activity. It lays the groundwork for human trials by assessing potential toxicity and pharmacological profiles.
Clinical Trials: Following successful preclinical results, the drug enters clinical trials, which are divided into several phases:
Submission of Applications: After successful Phase 3 trials, sponsors compile comprehensive documentation for the NDA or Biologics License Application (BLA). The quality and thoroughness of these submissions greatly affect the review timeline, with priority review applications averaging an 8-month processing time compared to 12 months for standard reviews.
Recent modifications in the FDA approval process, particularly in 2025, include the adoption of approaches such as artificial intelligence to expedite evaluations, potentially reducing timelines from 10-12 months to merely 1-2 months for specific applications. Furthermore, bioaccess® provides a distinctive sprint method, obtaining regulatory clearance in only 6-8 weeks, considerably quicker than the conventional 6-12 months observed in the US/EU. This accelerated method permits the enrollment of treatment-naive cardiology or neurology groups 50% quicker than Western locations, improving access to vital therapies.
Understanding these phases is vital for effectively navigating the FDA approval process. Proactive interaction with the FDA, including early consultations and scenario planning for various review results, can facilitate a smoother FDA approval process. Moreover, bioaccess's extensive clinical trial management services, encompassing feasibility studies and compliance evaluations, not only enhance the authorization process but also aid local economies through job creation and healthcare enhancements. As the landscape changes, keeping updated on the FDA approval process and requirements will enable innovators to foresee challenges and optimize their strategy for obtaining FDA clearance.
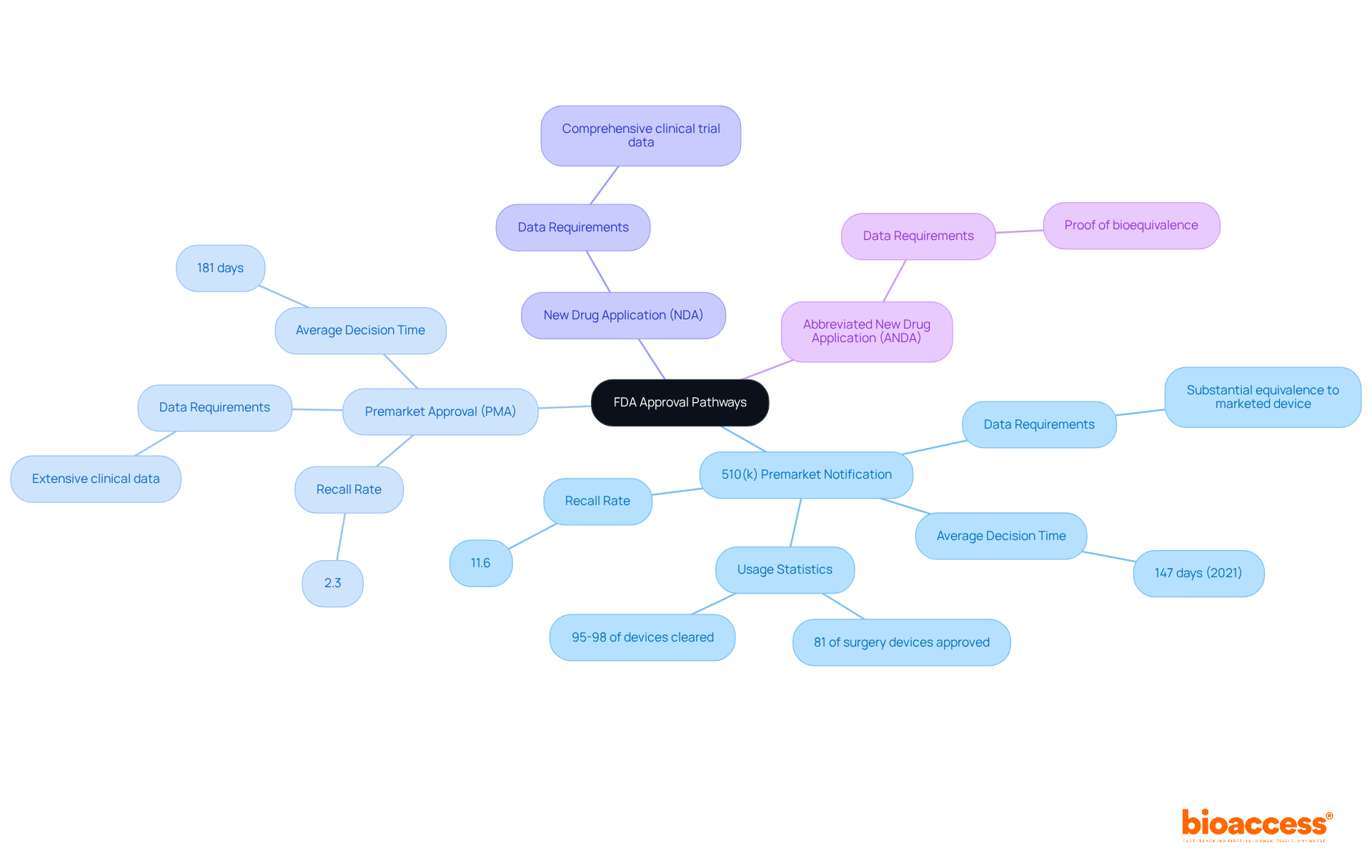
Navigating the FDA approval process requires a clear understanding of several key pathways, each tailored for specific product types. The primary pathways include:
510(k) Premarket Notification: This pathway is employed for devices that demonstrate substantial equivalence to an already marketed device. It generally necessitates less data and can be processed more swiftly, with an average decision time of about 147 days in 2021. Remarkably, approximately 95 to 98 percent of medical devices in the U.S. are authorized through this method. However, it's noteworthy that devices approved via 510(k) have a recall rate of 11.6%, significantly higher than the 2.3% recall rate for devices undergoing Premarket Approval (PMA).
Premarket Approval (PMA): This pathway is designated for novel devices or those lacking a predicate. It mandates extensive clinical data to substantiate safety and effectiveness, making it a more rigorous process. The average time for PMA decisions is around 181 days, reflecting the thorough evaluation required for high-risk devices. Recent trends indicate that while the number of PMA approvals has increased, the overall percentage of devices approved via 510(k) has decreased from 2002 to 2021, underscoring a shift in regulatory focus.
New Drug Application (NDA): This pathway is intended for new pharmaceuticals, necessitating comprehensive clinical trial data to support claims of safety and efficacy.
Abbreviated New Drug Application (ANDA): Utilized for generic drugs, this pathway requires proof of bioequivalence to an already approved drug.
Selecting the appropriate pathway is crucial and should align with the product's classification and intended use. Engaging with regulatory specialists can provide invaluable insights and guidance, particularly in light of the FDA approval process and its recent mandate for the use of the electronic Submission Template and Resource (eSTAR) for all 510(k) filings, effective October 1, 2023. This change aims to simplify the filing process and enhance overall efficiency.
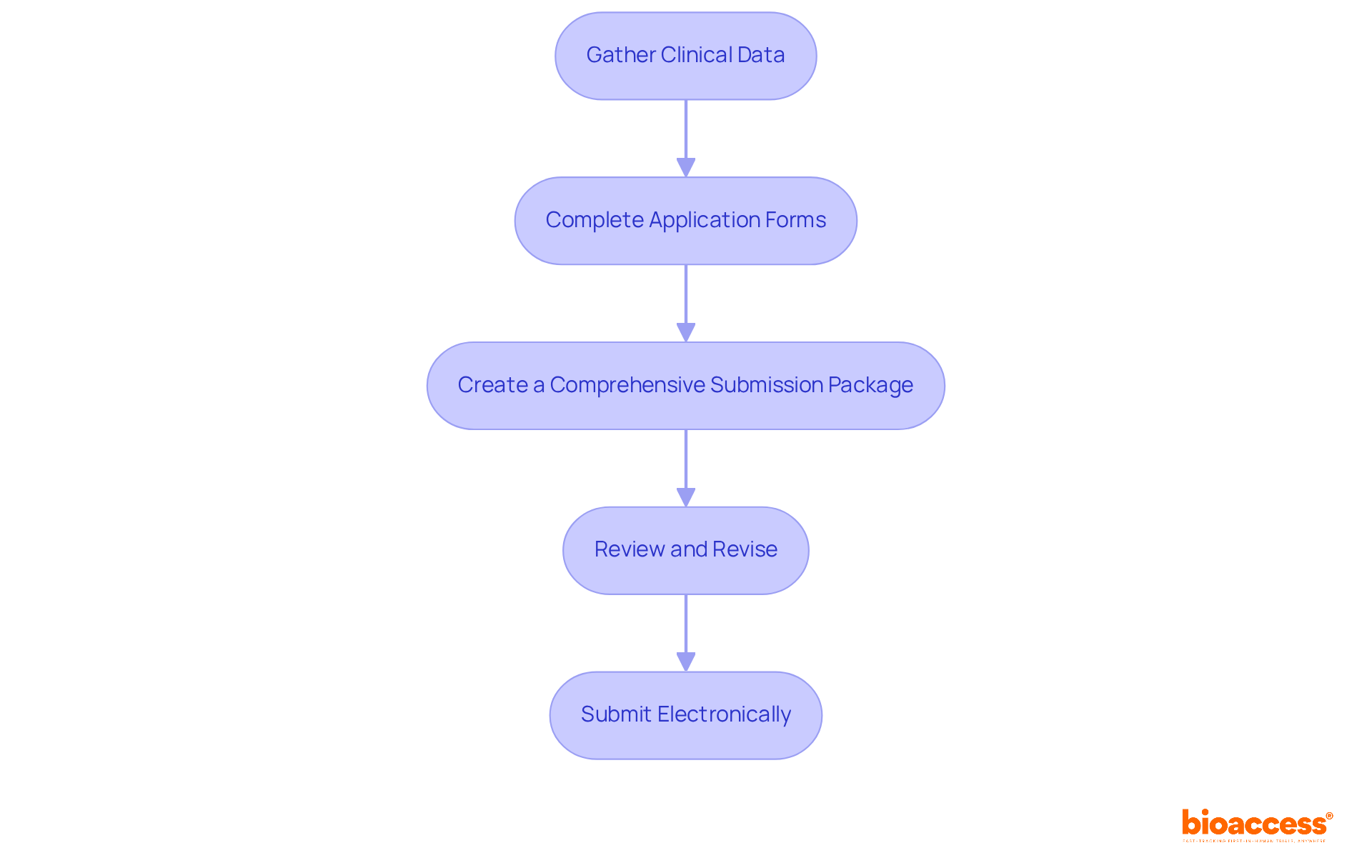
Preparing and submitting the required documentation involves several critical steps that are essential for a successful FDA application:
Gather Clinical Data: Compile all relevant clinical trial data, including study protocols, results, and statistical analyses. It is crucial to ensure that the data robustly supports the claims made in your application, as clinical data is paramount for the FDA approval process. In 2025, the necessity of comprehensive clinical data to substantiate safety and efficacy claims is emphasized by the FDA approval process.
Complete Application Forms: Fill out the necessary forms for your chosen pathway (e.g., 510(k), NDA). Each form has specific requirements that must be met, and understanding these nuances can significantly influence the success of your entry.
Create a Comprehensive Submission Package: This package should include all supporting documents, such as manufacturing methods, labeling, and risk assessments. Ensure that everything is organized and clearly labeled, as a well-structured presentation can facilitate a smoother review process.
Review and Revise: Prior to sending, conduct a thorough review of all documents to ensure accuracy and completeness. Engaging a regulatory expert for a final review can help identify potential issues, as initial rejections often stem from missing documents or incorrect information. Statistics indicate that up to 75% of first-time 510(k) applications are sent back, underscoring the importance of meticulous preparation.
Submit Electronically: Most entries are now completed electronically through the FDA's electronic filing system. Follow the guidelines for electronic entries to ensure a smooth process. The FDA's introduction of the Electronic Submission Template (eSTAR) has streamlined this aspect, making it essential to stay updated on protocol procedures.
By following these steps and prioritizing the quality of clinical data, companies can significantly improve their chances of successful filings in the FDA approval process.
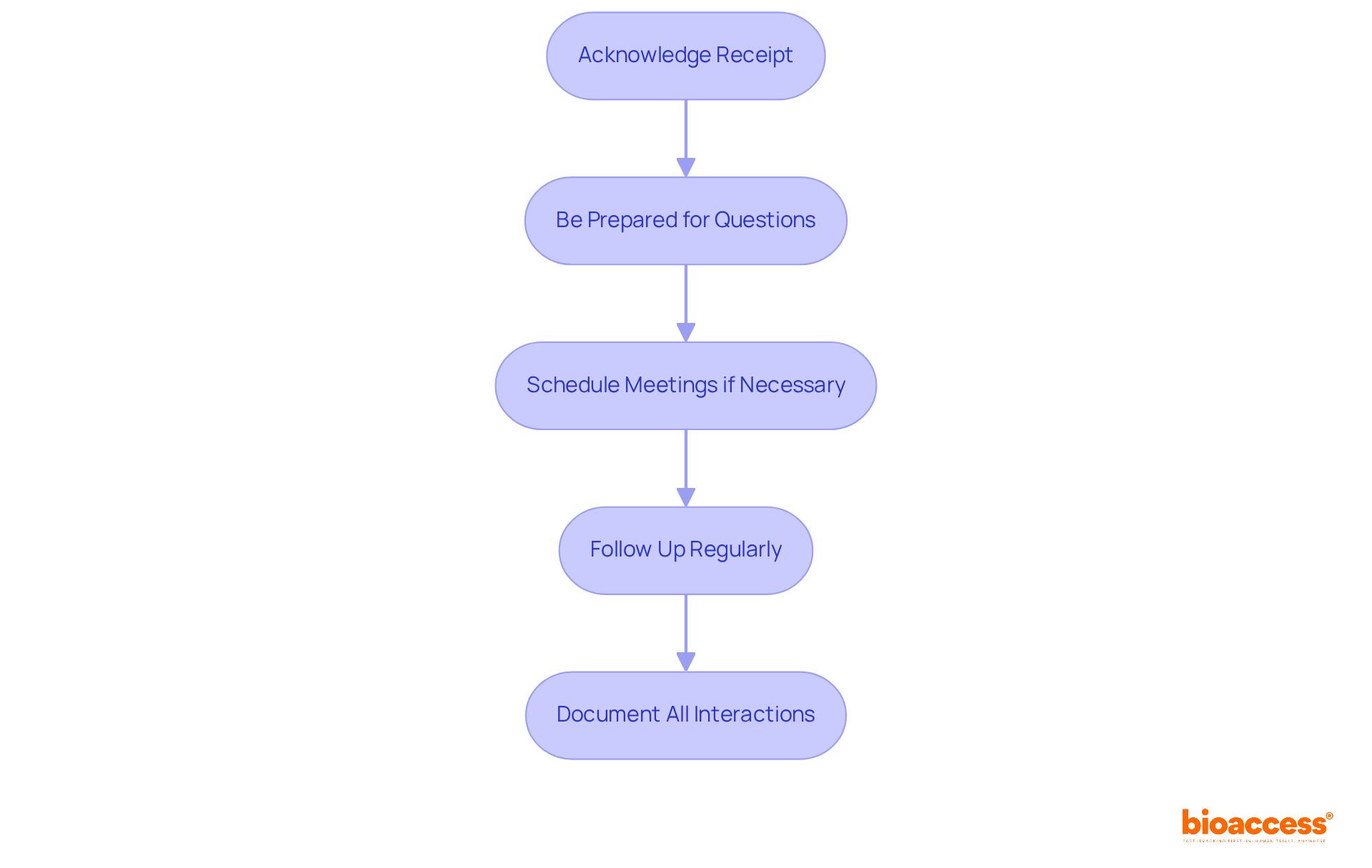
Effective communication with the FDA is crucial for a smooth FDA approval process following your application submission.
Statistics indicate that about 80 percent of FDA requests are addressed within 200 days, but some may take longer, highlighting the importance of proactive engagement. Effective communication strategies can significantly enhance the likelihood of a successful outcome, making it essential to prioritize these interactions throughout the FDA approval process.
Mastering the FDA approval process is essential for any organization seeking to bring new medications or medical devices to market. This intricate journey involves multiple stages, from preclinical testing to clinical trials, culminating in the submission of critical applications like the NDA or BLA. Each phase presents unique challenges and requirements that must be navigated with precision to ensure a successful outcome.
Key points highlighted throughout the article include:
As the landscape of FDA approvals continues to evolve, staying informed and proactive is paramount. Engaging with regulatory specialists and maintaining open lines of communication with the FDA can greatly enhance the likelihood of a successful application. For innovators in the healthcare field, embracing these strategies not only optimizes the path to regulatory clearance but also ultimately contributes to the timely delivery of vital therapies to patients in need.
What is the purpose of the FDA approval process?
The FDA approval process aims to guarantee the safety and effectiveness of new medications and medical instruments before they enter the market.
What are the main stages of the FDA approval process?
The main stages include preclinical testing, clinical trials, and the submission of important applications such as the Investigational New Drug (IND) application and the New Drug Application (NDA).
What happens during the preclinical testing phase?
Preclinical testing involves laboratory and animal studies to gather data on a drug's safety and biological activity, assessing potential toxicity and pharmacological profiles before human trials.
How are clinical trials structured?
Clinical trials are divided into three phases: Phase 1: Focuses on safety and dosage with 20 to 100 participants. Phase 2: Involves 100 to 300 participants to further assess efficacy and monitor side effects. Phase 3: Confirms safety and efficacy in a larger population, often involving 1,000 to 3,000 subjects.
What is the significance of the NDA or Biologics License Application (BLA)?
After successful Phase 3 trials, sponsors compile documentation for the NDA or BLA, which is crucial for the review process and can significantly affect the timeline for approval.
How do recent modifications in the FDA approval process impact timelines?
Recent modifications, particularly in 2025, include the use of artificial intelligence to expedite evaluations, potentially reducing review timelines from 10-12 months to 1-2 months for specific applications.
What is bioaccess® and how does it differ from traditional approval methods?
Bioaccess® employs a sprint method to obtain regulatory clearance in only 6-8 weeks, significantly faster than the conventional 6-12 months observed in the US/EU.
How can proactive interaction with the FDA benefit the approval process?
Proactive interaction, including early consultations and scenario planning, can facilitate a smoother FDA approval process and help innovators navigate challenges effectively.
What additional services does bioaccess provide to enhance the approval process?
Bioaccess offers extensive clinical trial management services, including feasibility studies and compliance evaluations, which enhance the authorization process and contribute to local economies through job creation and healthcare improvements.
Why is it important to stay updated on the FDA approval process?
Keeping updated on the FDA approval process and requirements enables innovators to foresee challenges and optimize their strategies for obtaining FDA clearance.