


The FDA approval process for medical devices serves as a structured pathway that guarantees safety and effectiveness. This process encompasses essential stages such as:
Understanding the specific pathways—such as 510(k), PMA, and De Novo classification—is crucial. Strategic planning and early collaboration with the FDA significantly enhance the likelihood of successful device approval and compliance. By recognizing these elements, stakeholders can navigate the complexities of the approval process more effectively.
Navigating the FDA approval process for medical devices represents a critical venture for manufacturers seeking to introduce innovative healthcare solutions to the market. This complex journey not only guarantees that products adhere to stringent safety and efficacy standards but also provides a strategic roadmap for compliance, which can significantly impact a company’s success.
As the medical technology landscape continues to evolve, grasping the nuances of pathways such as:
is essential. How can companies effectively position themselves to meet regulatory requirements while leveraging these as catalysts for innovation and market entry?
The FDA approval process for medical devices represents a crucial pathway that ensures safety and effectiveness before products reach the market. This multifaceted process encompasses several stages, including pre-market submissions, thorough reviews, and ongoing post-market surveillance. A comprehensive understanding of the FDA approval process for medical devices is essential for effectively navigating the intricate landscape of regulatory compliance. Key components involve:
Notably, approximately 70% of medical devices successfully navigate the FDA approval process for medical devices, which underscores the importance of strategic planning and implementation.
Collaborating with the FDA early in the development phase can yield invaluable insights and guidance, significantly streamlining the FDA approval process for medical devices and your submission and clearance timeline. Recent updates to FDA regulations reflect a commitment to adapting to technological advancements and emerging health concerns, making it essential for companies to remain informed. For instance, implementing a robust document and quality management system (QMS) can mitigate errors and delays in regulatory submissions, thereby enhancing compliance.
Real-world examples illustrate the effectiveness of proactive engagement with regulatory bodies. Businesses that adopt a strategic approach to regulatory adherence not only accelerate their validation timelines but also reinforce their commitment to providing safe and effective products. As a leading regulatory expert observes, viewing compliance as an ongoing commitment to patient safety and product quality transforms regulatory challenges into opportunities for innovation and growth.

The FDA approval process for medical devices offers three primary routes for equipment clearance:
The 510(k) procedure is typically used for products demonstrating significant similarity to existing items, allowing for a quicker authorization timeline, often averaging around 3 to 6 months. In contrast, the FDA approval process for medical devices is mandated for high-risk products and entails a more rigorous review process, including comprehensive clinical trials, which can span 1 to 3 years for validation. The De Novo pathway is designed for new products lacking a predicate, categorizing them as low to moderate risk, and generally offers an efficient process that can be completed in approximately 6 months to a year.
Recent updates in the FDA approval process for medical devices aim to enhance the efficiency of these pathways, particularly in response to the increasing demand for innovative medical technologies. For instance, the FDA has initiated programs to expedite the evaluation of innovative products, significantly reducing assessment times for qualifying items.
Case studies effectively illustrate the distinctions among these pathways: a prominent example is the approval of the SolePower Work Boots, which employed the 510(k) process due to its substantial equivalence to existing safety footwear, leading to a rapid market entry. Conversely, a high-risk product like the Medtronic Cardioblate® necessitated PMA, highlighting the extensive clinical data required to establish safety and efficacy.
Expert opinions underscore the importance of selecting the appropriate route based on the device's risk profile and intended use. Grasping these differences not only aids in regulatory compliance but also fosters a more strategic approach to market entry, ultimately increasing the likelihood of successful commercialization.

To prepare your submission for the FDA approval process for medical devices, begin by gathering all essential documentation, including:
For a 510(k) submission, it is crucial to demonstrate substantial equivalence to a predicate product. In the case of a PMA, comprehensive clinical data and risk assessments are mandatory. Ensure that all documents comply with the FDA approval process for medical devices, including both formatting and content requirements.
Conducting a pre-submission meeting with the FDA is advisable to clarify expectations and obtain feedback on your submission strategy related to the FDA approval process for medical devices. This proactive approach can significantly enhance your chances of approval.

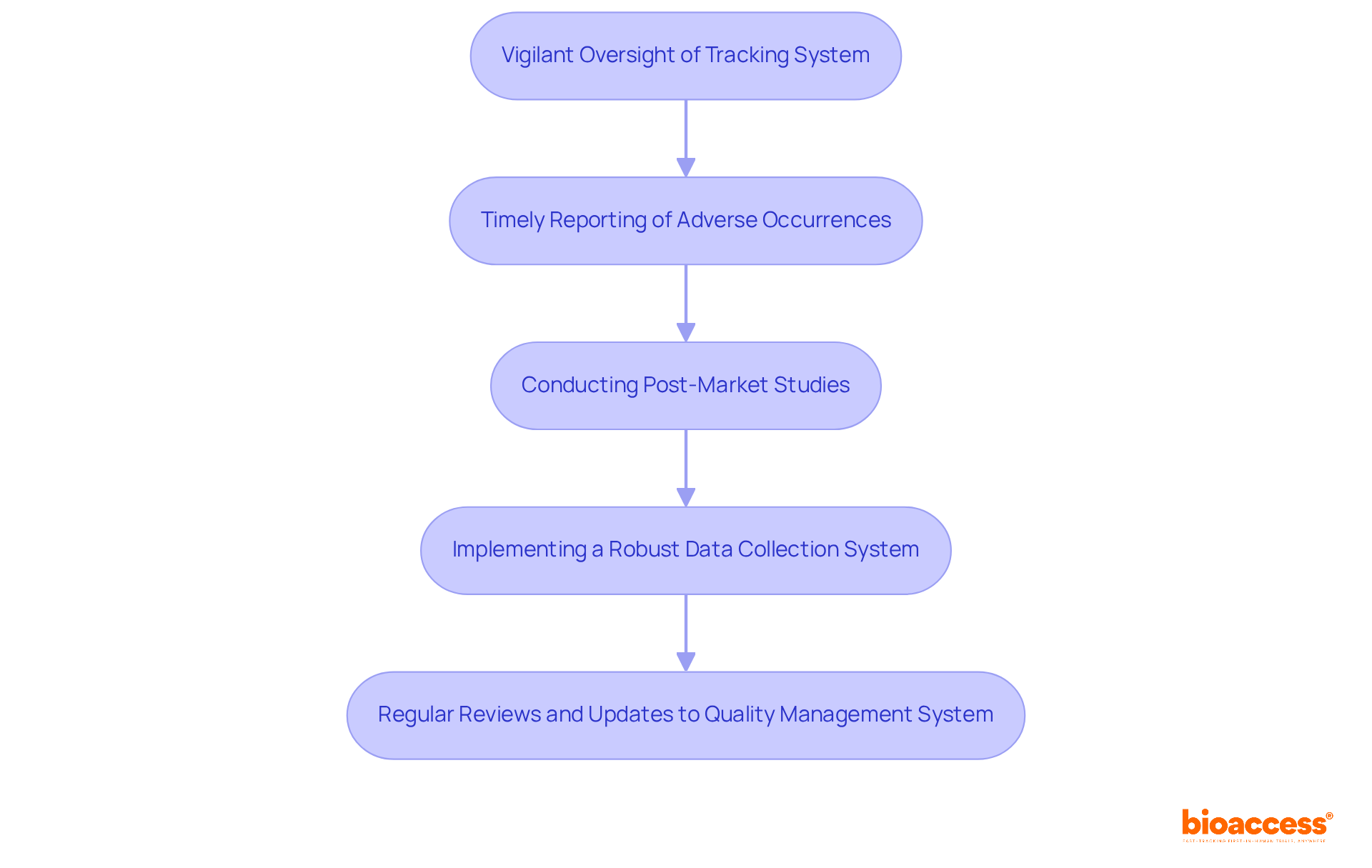
Once your medical equipment is approved through the FDA approval process for medical devices, maintaining compliance with FDA regulations is paramount through ongoing monitoring and reporting. This involves:
Manufacturers must implement a robust system for collecting and analyzing data pertinent to device safety and effectiveness. Regular reviews and updates to your quality management system (QMS) are crucial to ensure continued compliance with the FDA approval process for medical devices, enabling swift resolution of any issues that may arise. Furthermore, engaging with healthcare professionals and users can yield invaluable feedback that informs future enhancements and compliance initiatives.
Navigating the FDA approval process for medical devices is essential for ensuring that products are safe and effective before reaching consumers. Mastering this intricate journey requires a thorough understanding of key pathways, meticulous documentation preparation, and unwavering compliance throughout the product lifecycle. By strategically approaching each stage of the process, manufacturers can enhance their chances of successful approval while fostering a culture of safety and innovation within the medical device industry.
This article highlights several critical components of the FDA approval process, including:
It emphasizes the value of early engagement with the FDA during development and outlines the distinct pathways—510(k), PMA, and De Novo—each tailored to different risk profiles and product types. Furthermore, it underscores the necessity of ongoing compliance and post-market surveillance to ensure continued safety and effectiveness after approval.
Ultimately, understanding the FDA approval process for medical devices transcends mere regulatory compliance; it represents a steadfast commitment to patient safety and product quality. As the landscape of medical technology evolves, staying informed about the latest FDA guidelines and adapting to new challenges will be crucial. By prioritizing strategic planning and collaboration with regulatory bodies, companies can transform potential obstacles into opportunities, driving innovation while safeguarding public health.
What is the FDA approval process for medical devices?
The FDA approval process for medical devices is a crucial pathway that ensures the safety and effectiveness of products before they reach the market. It includes several stages such as pre-market submissions, thorough reviews, and ongoing post-market surveillance.
Why is it important to understand the FDA approval process?
A comprehensive understanding of the FDA approval process is essential for navigating the complex landscape of regulatory compliance, which can help ensure that medical devices meet safety and effectiveness standards.
What are the key components of the FDA approval process?
Key components of the FDA approval process include identifying the category of the medical equipment, preparing the necessary documentation, and adhering to established timelines.
What percentage of medical devices successfully navigate the FDA approval process?
Approximately 70% of medical devices successfully navigate the FDA approval process, highlighting the importance of strategic planning and implementation.
How can collaboration with the FDA benefit the approval process?
Collaborating with the FDA early in the development phase can provide invaluable insights and guidance, significantly streamlining the approval process and improving submission and clearance timelines.
What recent updates have been made to FDA regulations?
Recent updates to FDA regulations reflect a commitment to adapting to technological advancements and emerging health concerns, making it essential for companies to stay informed about these changes.
How can a robust document and quality management system (QMS) impact regulatory submissions?
Implementing a robust document and quality management system (QMS) can help mitigate errors and delays in regulatory submissions, thereby enhancing compliance with FDA regulations.
What is the significance of proactive engagement with regulatory bodies?
Proactive engagement with regulatory bodies allows businesses to accelerate their validation timelines and reinforces their commitment to providing safe and effective products.
How can viewing compliance as an ongoing commitment benefit companies?
Viewing compliance as an ongoing commitment to patient safety and product quality can transform regulatory challenges into opportunities for innovation and growth.