


The FDA drug approval process can be mastered by following four key steps:
This article outlines these steps in detail, emphasizing the importance of:
By mastering these steps, stakeholders can significantly improve their chances of navigating the complexities of drug approval.
Navigating the FDA drug approval process is a crucial journey that ensures new medications are safe and effective before reaching patients. While it may seem daunting, this guide breaks down the intricate framework into four manageable steps, offering valuable insights into:
In a landscape marked by evolving regulations and increasing competition, stakeholders must consider how to effectively streamline their approach to enhance their chances of success in this rigorous environment.
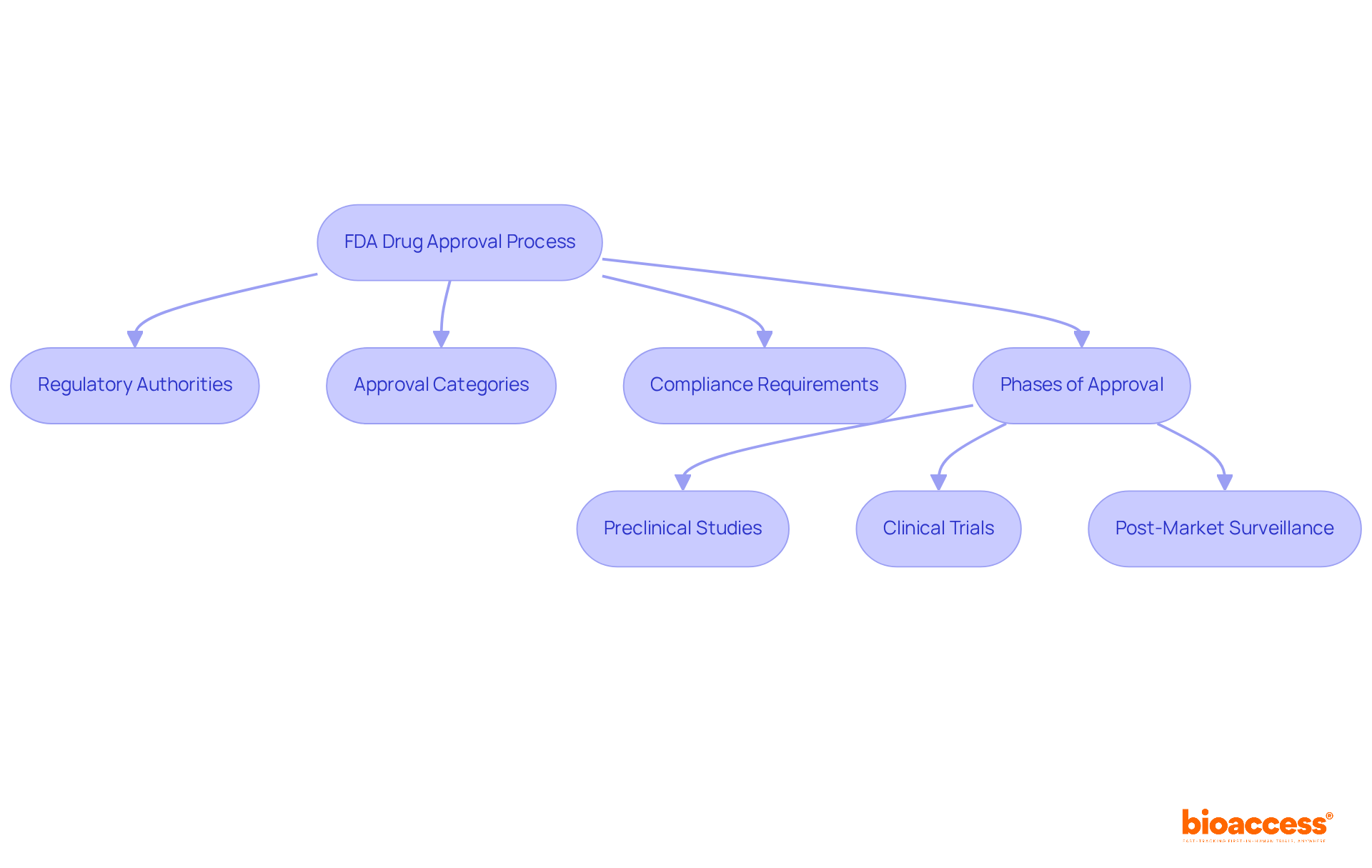
The fda drug approval process represents a meticulously structured pathway designed to ensure that new substances are both safe and effective prior to market entry. This process encompasses several critical components:
Regulatory Authorities: The FDA operates through multiple centers, with the Center for Evaluation and Research (CDER) leading the assessment of new medications. This division is essential for upholding strict standards in medication safety and efficacy.
Approval Categories: Drugs are categorized into new molecular entities (NMEs), biologics, or generics, each following distinct approval pathways. This classification is vital for understanding the specific regulatory requirements and timelines associated with each type.
Compliance Requirements: Adherence to Good Clinical Practice (GCP) and Good Manufacturing Practice (GMP) is crucial throughout the medication development process. These standards ensure that clinical trials are conducted ethically and that manufacturing processes meet quality and compliance benchmarks.
Phases of the FDA Drug Approval Process: Understanding the stages of medication approval is essential. This encompasses preclinical studies, which examine effectiveness and security in laboratory environments, succeeded by clinical trials that assess the substance's performance in human participants. Post-market surveillance also plays a key role, monitoring the medication's long-term safety and effectiveness once it is available to the public.
Recent statistics reveal that from 2013 to 2022, 428 medications were sanctioned by the FDA, with 87% of these authorizations occurring in the initial review cycle, underscoring the growing efficiency of the process. Moreover, the FDA's accelerated programs, such as the Breakthrough Therapy designation, have significantly shortened authorization timeframes, with priority evaluations averaging about 8 months compared to the typical 12 months.
Industry leaders emphasize the importance of strategic planning and proactive interaction with regulatory authorities to enhance the authorization process. For example, early FDA engagement through pre-NDA meetings can align expectations and identify potential review issues, ultimately leading to a smoother submission process.
By grasping these foundational elements, stakeholders can better navigate the complexities of the FDA drug approval process and enhance their chances of successful development.
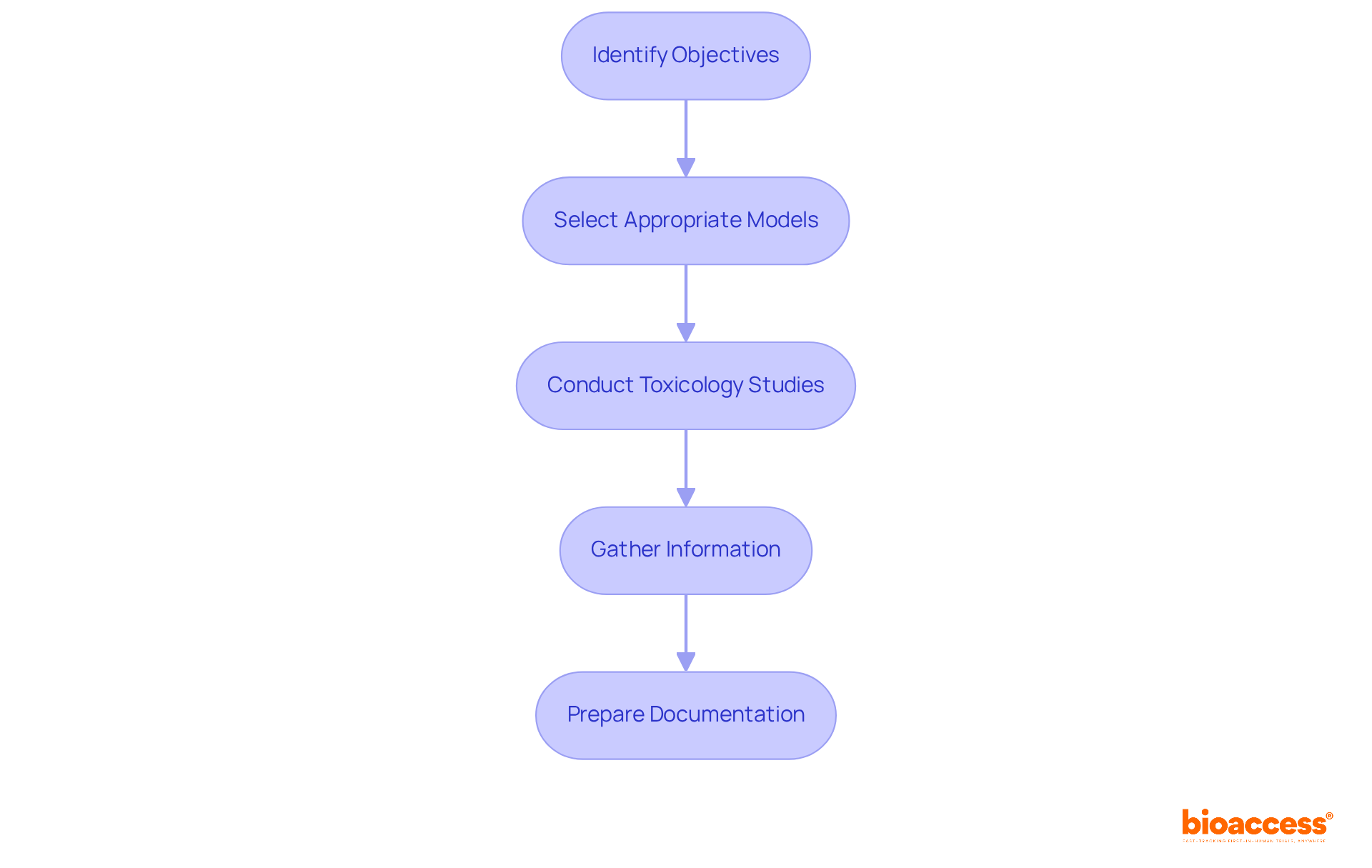
Preclinical research is a critical phase that involves laboratory and animal investigations to assess the safety and biological effects of a candidate compound. To conduct effective preclinical research, follow these essential steps:
By meticulously following these steps, researchers can establish a solid foundation for the subsequent phases of clinical trials.
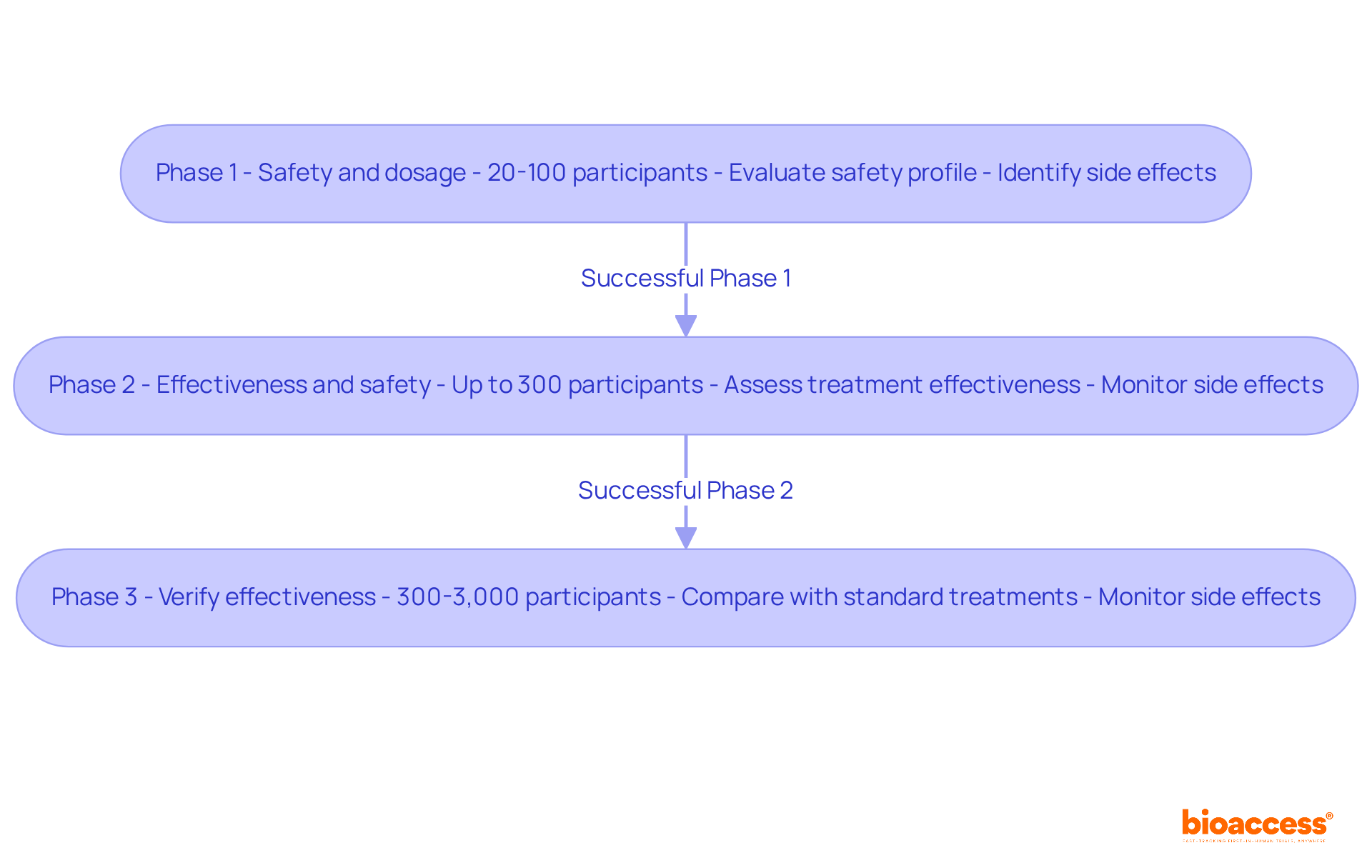
Clinical trials are structured into three primary phases, each targeting specific aspects of a drug's safety and efficacy.
Phase 1: This initial phase is crucial for establishing security. A small group of healthy volunteers, typically ranging from 20 to 100 participants, receives the medication. The main goals are to evaluate the medication's safety profile, establish suitable dosage ranges, and recognize any possible side effects. Approximately 70 percent of experimental medications successfully pass through Phase 1, despite the inherent risks involved. These trials are essential for patients with life-threatening conditions who have exhausted other treatment options, providing early access to innovative therapies.
Phase 2: Following successful Phase 1 trials, the medication is administered to a larger group of patients, usually up to 300, who have the condition the treatment is intended to address. The primary emphasis during this phase is to assess the treatment's effectiveness while continuing to monitor its safety. This phase often involves evaluating whether the treatment can reduce tumors or enhance quality of life, determining if the medication justifies further testing in Phase 3.
Phase 3: This phase significantly expands the participant pool, involving between 300 and 3,000 patients. The aim is to verify the medication's effectiveness, observe side effects, and compare it against standard treatments. Phase 3 trials are essential for the New Drug Application (NDA) submission, as successful completion can lead to the FDA drug approval process for wider use. Participants are often randomly assigned to receive either the new treatment or the standard therapy, ensuring a robust comparison of outcomes.
Each phase is interconnected, building upon the findings of the previous one. Rigorous planning and execution are essential to facilitate a seamless transition through the clinical trial process. With bioaccess®'s comprehensive clinical trial management services, including feasibility studies, compliance reviews, and site selection, the process is streamlined. This allows for accelerated patient enrollment—50% faster for cardiology and neurology cohorts—resulting in significant cost savings of $25K per patient. This efficiency not only enhances the trial's success rate but also contributes positively to local economies through job creation and healthcare improvements, ultimately leading to the advancement of new therapies that can significantly impact patient care.
The New Drug Application (NDA) serves as the official petition to the FDA in the FDA drug approval process for authorization to promote a new medication. Understanding the steps to prepare and submit an NDA is crucial for success in the clinical research landscape:
The FDA drug approval process is crucial for ensuring the safety and efficacy of new medications. After submission, the FDA drug approval process gives the FDA 60 days to decide whether to file the NDA for review. The FDA drug approval process typically takes 6-10 months, during which the FDA evaluates the data and may request additional information.
Successfully navigating this final step is essential for obtaining the FDA drug approval process and bringing the drug to market.

Mastering the FDA drug approval process is essential for stakeholders aiming to bring new medications to market successfully. By understanding the structured framework that governs this process—including the critical roles of regulatory authorities, compliance requirements, and the various approval categories—stakeholders can navigate the complexities involved with greater confidence.
This article details the four key steps in the FDA drug approval journey:
Each step builds upon the last, emphasizing the importance of strategic planning and proactive engagement with regulatory bodies. The insights provided highlight the necessity of rigorous testing and documentation to ensure that new drugs are both safe and effective.
Ultimately, the FDA drug approval process is not just a bureaucratic hurdle; it serves as a vital safeguard that protects public health. As the landscape of drug development continues to evolve, stakeholders are encouraged to stay informed about FDA regulations, streamline their research methodologies, and engage in early dialogue with the FDA. By doing so, they can enhance their chances of successfully navigating this essential process and contribute to the advancement of innovative therapies that can significantly improve patient care.
What is the purpose of the FDA drug approval process?
The FDA drug approval process is designed to ensure that new substances are both safe and effective before they enter the market.
Which regulatory authority leads the assessment of new medications?
The Center for Evaluation and Research (CDER) within the FDA leads the assessment of new medications.
What are the different approval categories for drugs?
Drugs are categorized into new molecular entities (NMEs), biologics, or generics, each following distinct approval pathways.
Why are compliance requirements important in the drug approval process?
Compliance with Good Clinical Practice (GCP) and Good Manufacturing Practice (GMP) is crucial to ensure ethical conduct in clinical trials and that manufacturing processes meet quality and compliance standards.
What are the main phases of the FDA drug approval process?
The main phases include preclinical studies to assess effectiveness and safety in laboratories, followed by clinical trials in human participants, and post-market surveillance to monitor long-term safety and effectiveness.
How many medications were sanctioned by the FDA from 2013 to 2022?
From 2013 to 2022, the FDA sanctioned 428 medications.
What percentage of FDA authorizations occurred in the initial review cycle from 2013 to 2022?
87% of the FDA authorizations occurred in the initial review cycle during that period.
What is the significance of the FDA's accelerated programs?
The FDA's accelerated programs, such as the Breakthrough Therapy designation, have significantly shortened authorization timeframes, with priority evaluations averaging about 8 months compared to the typical 12 months.
How can industry leaders enhance the drug approval process?
Industry leaders can enhance the drug approval process through strategic planning and proactive interaction with regulatory authorities, such as engaging early with the FDA through pre-NDA meetings to align expectations and identify potential review issues.