


The FDA pre-submission process is essential for companies seeking guidance on their medical device or drug applications. This process facilitates early communication with the FDA, allowing for the identification of potential submission issues. Companies must:
These steps are crucial for improving the chances of regulatory success and expediting market authorization.
Navigating the FDA pre-submission process presents a formidable challenge for companies eager to bring their medical devices or drugs to market. This critical phase serves as a unique opportunity for sponsors to engage with the FDA, clarify submission requirements, and refine their regulatory strategies, ultimately enhancing their chances of success. However, with approximately two-thirds of pre-submission requests involving discussions with the FDA, the stakes are high.
What occurs when these interactions are mismanaged or overlooked? Understanding the nuances of the pre-submission process is essential for any sponsor aiming to avoid common pitfalls and leverage the insights gained for a smoother regulatory journey.
The fda pre submission process is essential for companies seeking insights and guidance from the FDA before formally submitting their applications for medical devices or drugs. This process enables sponsors to present their proposed study designs, seek input on regulatory strategies, and clarify submission requirements. Key components of this process include:
At bioaccess®, we provide comprehensive clinical trial management services encompassing feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Our expertise in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), positions us to effectively support your regulatory journey. Additionally, we offer review and feedback on study documents to ensure compliance with country requirements, further enhancing the quality of your submission.
Mastering these foundational elements equips you to manage the process before submission efficiently, thereby improving your chances of regulatory success. Engaging in the FDA pre submission process is particularly advantageous, as approximately two-thirds of requests before submission involve discussions with the FDA, which can significantly expedite the path to market authorization. Effective utilization of these interactions can establish a strong foundation for successful submissions, as evidenced by case studies highlighting the importance of early involvement and thorough preparation. Furthermore, it is crucial to note that there are no user fees associated with pre-submissions, making this process a cost-effective strategy for sponsors. By understanding and applying these principles, you can better prepare for the next steps in your regulatory journey.
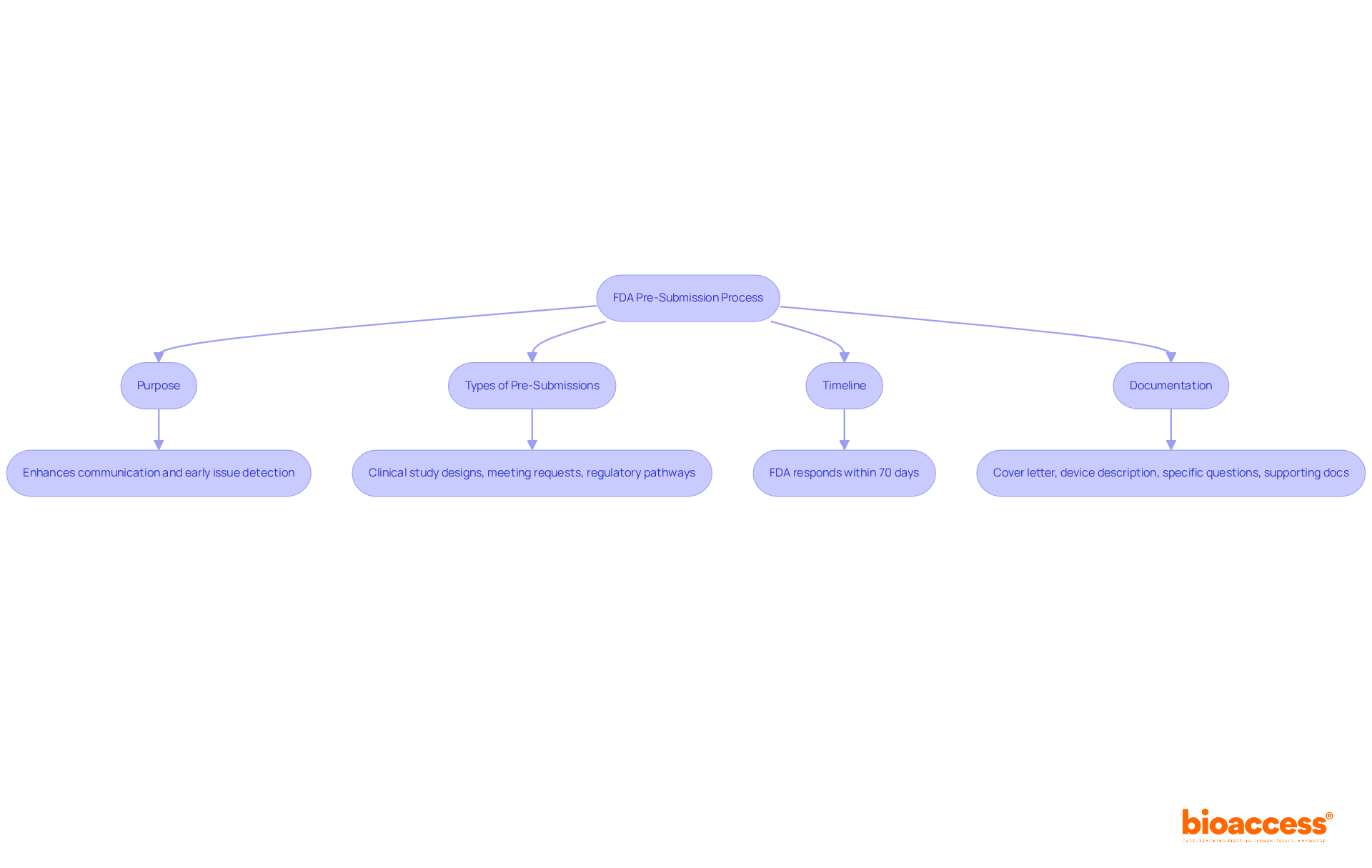
To prepare an effective pre-submission request, follow these steps:
By meticulously preparing your request for the FDA pre submission, you create the opportunity for a productive dialogue with the FDA, thereby increasing the likelihood of a favorable outcome. Notably, 89% of PMA submissions are rejected the first time they are submitted, underscoring the importance of thorough preparation. Furthermore, understanding the media coverage of clinical trials in Latin America, particularly in Colombia, can provide valuable insights into the regulatory environment and enhance your strategic approach.
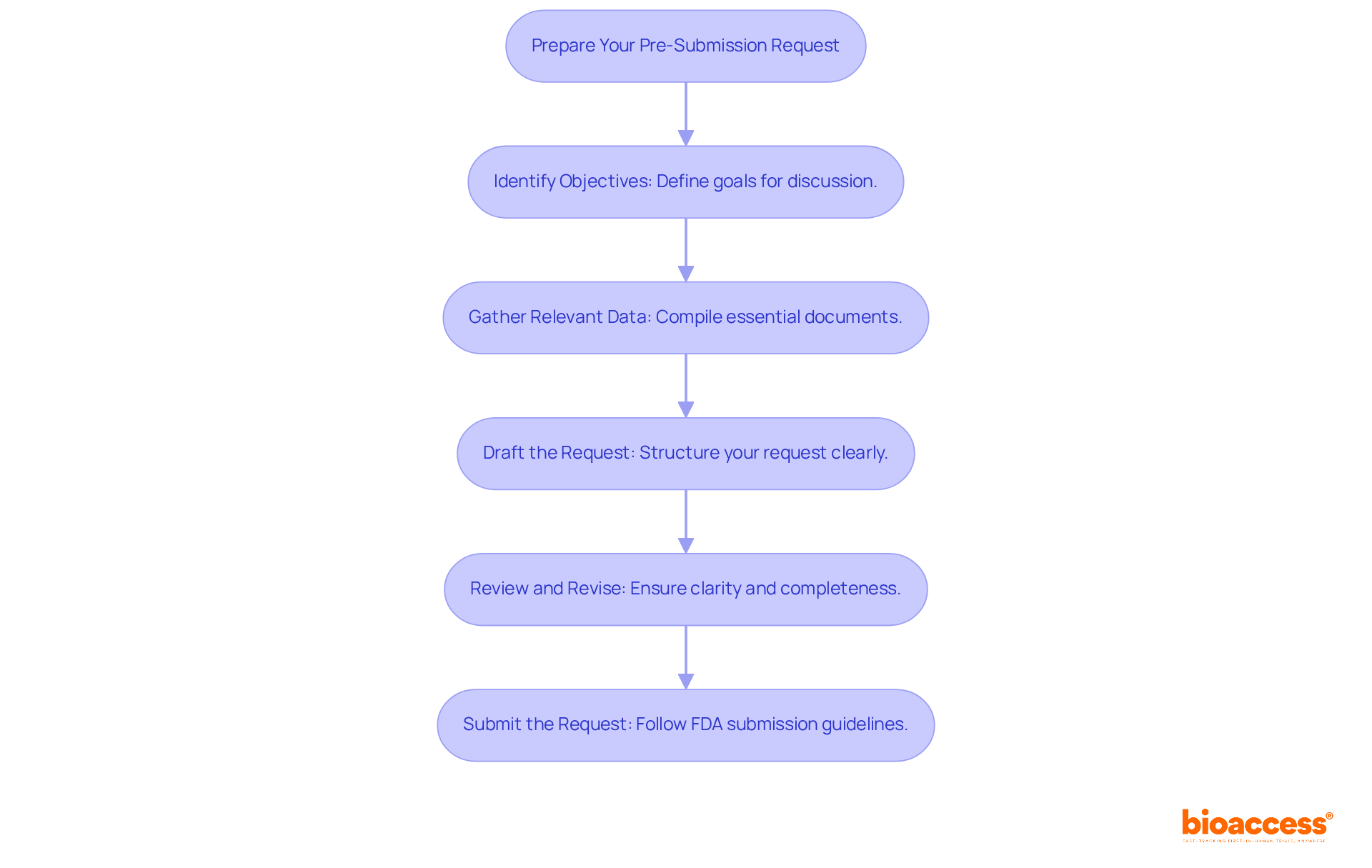
To ensure a successful pre-submission meeting, it is crucial to avoid these common pitfalls:
Lack of Preparation: Inadequate preparation can lead to missed opportunities for valuable feedback. Align all team members on objectives and questions well in advance, ideally starting three to six months before the gathering.
Overloading with Information: Providing excessive information can overwhelm FDA reviewers. Focus on key points and questions that directly relate to your objectives, ensuring clarity and conciseness in your presentation.
Ignoring FDA Guidance: Not adhering to FDA guidelines can create ambiguity in your request. Acquaint yourself with the FDA pre submission expectations for discussions, which include the required timelines for submitting packet materials—at least 30 days in advance for Type B sessions.
Failure to Follow Up: Neglecting to follow up on action items or feedback after the gathering can stall progress. Record the outcomes of the discussion and ensure all team members are aware of the next steps to sustain momentum.
Not Engaging the Right Stakeholders: Ensure that the appropriate team members, including regulatory affairs specialists and clinical researchers, are present during the discussion. Their expertise is vital for addressing specific questions effectively and demonstrating a comprehensive understanding of the product.
By steering clear of these common mistakes, you can significantly enhance the quality of your interactions with the FDA, ultimately increasing the likelihood of a successful outcome.
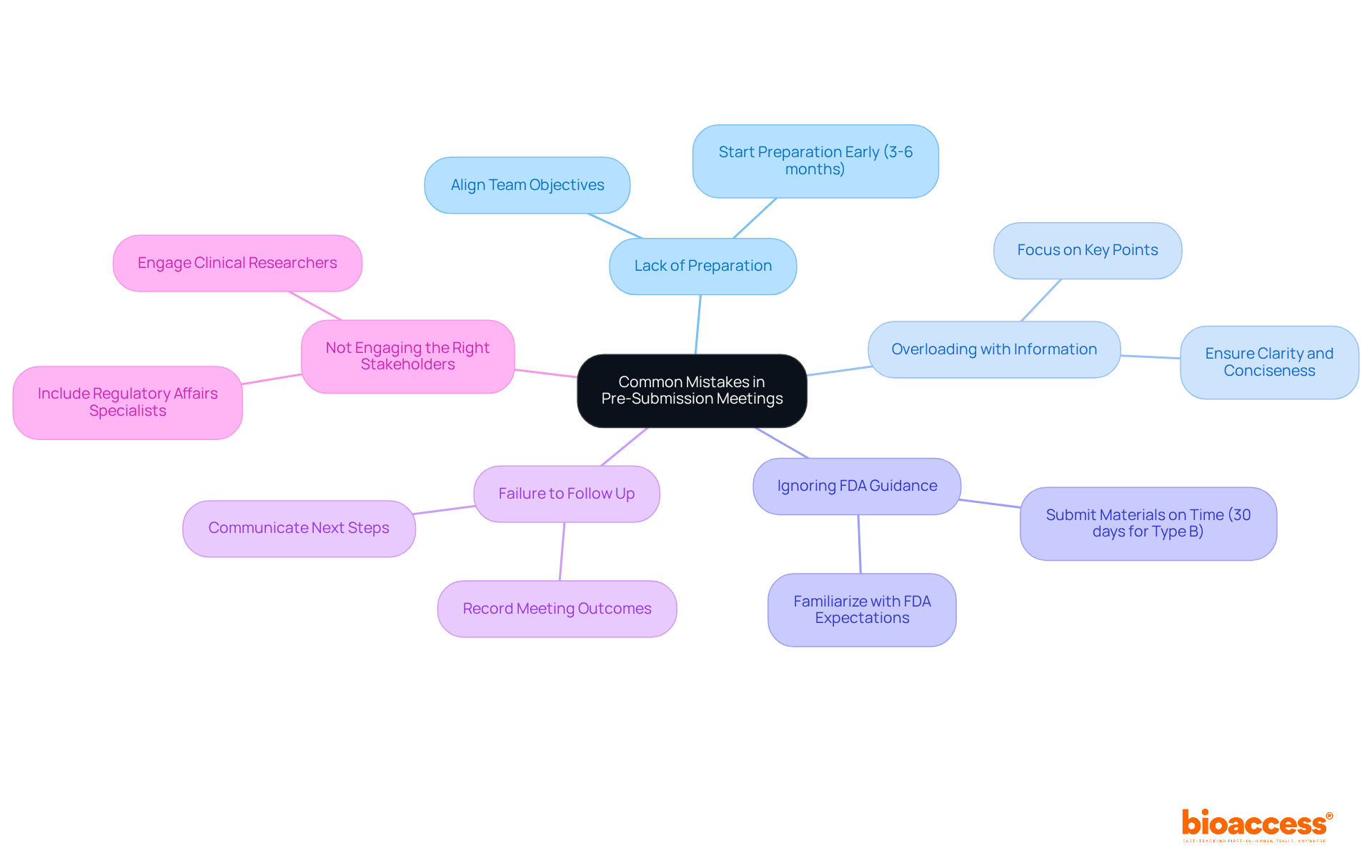
Following your FDA pre submission discussion, it is crucial to efficiently implement the suggestions and detail your forthcoming actions. Here’s a structured approach to guide you:
Mike Drues emphasizes, "The biggest advantage of having an FDA pre submission is greatly mitigating if not completely eliminating, those statistics I just shared with you." By systematically implementing feedback and outlining clear next steps, you can significantly enhance your project’s trajectory and ensure compliance with FDA expectations. Case studies illustrate that well-designed action plans following initial discussions can lead to more favorable outcomes, as demonstrated in the case study titled 'The Importance of Initial Discussions,' which highlights how efficient action plans can facilitate the regulatory process. Additionally, be wary of common pitfalls, such as neglecting to ask leading questions that guide the FDA towards the desired conclusions. By avoiding these pitfalls and preparing thoroughly, companies can improve their chances of success in FDA pre submission meetings, particularly when supported by bioaccess®'s innovative approach to clinical research in Latin America. Furthermore, utilizing bioaccess®'s services can yield significant cost savings, with a cost-to-speed ratio that allows for $25K savings per patient, thereby enhancing the overall efficiency of the clinical trial process.
Navigating the FDA pre-submission process is a critical step for companies aiming to ensure a smooth regulatory journey for their medical devices or drugs. Engaging with the FDA early allows sponsors to clarify submission requirements, refine study designs, and mitigate potential issues that could hinder their path to market authorization.
This article outlines essential components of the pre-submission process, including:
Key strategies such as:
are emphasized as vital for enhancing the chances of success. Additionally, the absence of user fees for pre-submissions makes this process a cost-effective option for many companies.
Ultimately, mastering the FDA pre-submission process not only streamlines regulatory compliance but also fosters productive dialogues with the FDA that can lead to more favorable outcomes. Companies are encouraged to leverage insights gained from these interactions and continuously improve their submission strategies. By prioritizing preparation and actively engaging with regulatory experts, sponsors can significantly enhance their prospects for success in the competitive landscape of medical device and drug approval.
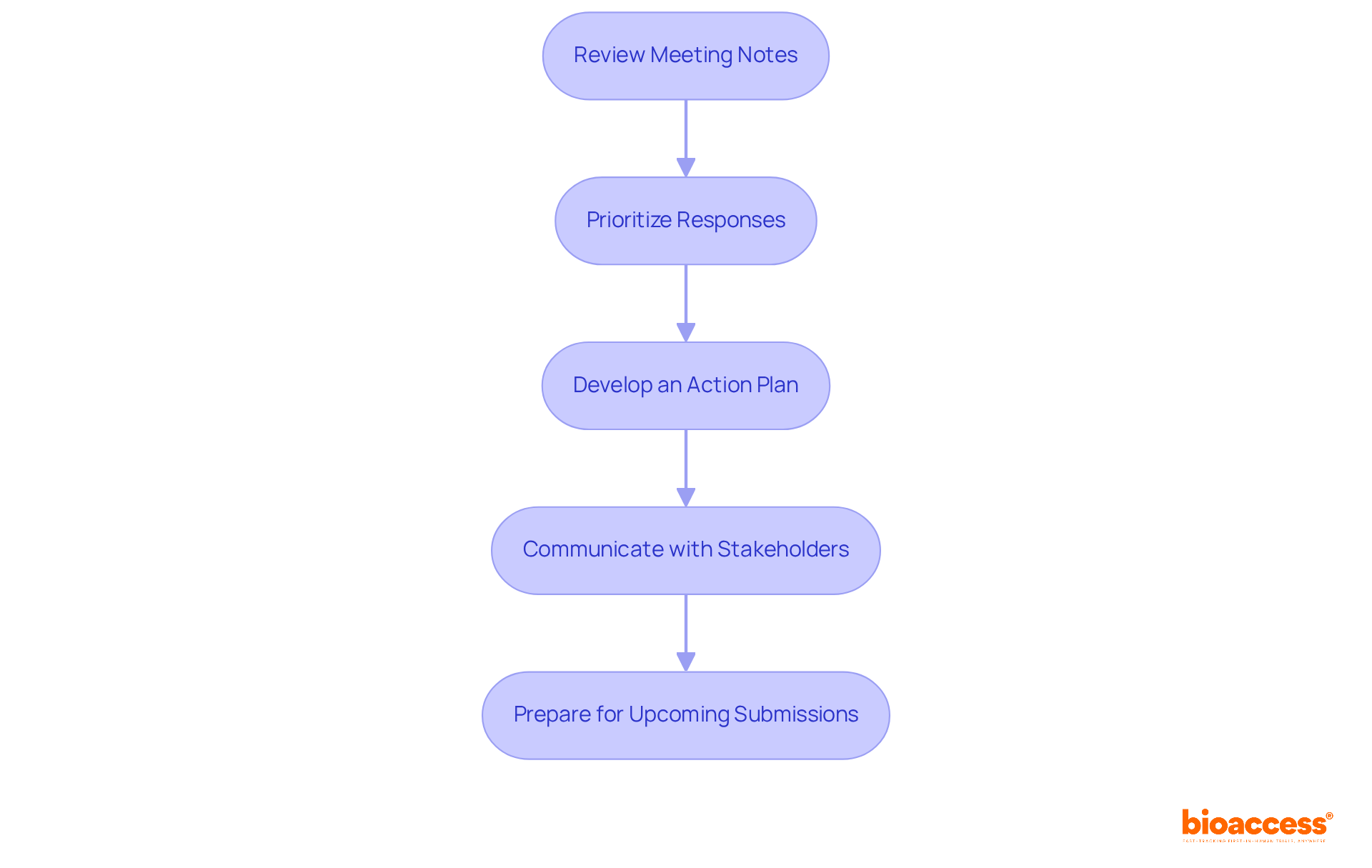
What is the purpose of the FDA pre-submission process?
The FDA pre-submission process enhances communication between the FDA and sponsors, allowing for early detection of potential issues that could impact the submission of medical devices or drugs.
What types of pre-submissions are available?
Various categories of pre-submissions exist, including requests for input on clinical study designs, meeting requests, and inquiries about specific regulatory pathways.
How long does it typically take for the FDA to respond to pre-submission requests?
The FDA typically aims to respond to preliminary requests within 70 days, allowing sponsors to adjust their strategies based on the feedback received.
What documentation is required for a pre-submission?
A meticulously assembled package is crucial and should include a cover letter, device description, specific questions, and supporting documentation.
How can bioaccess® assist with the FDA pre-submission process?
Bioaccess® provides comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, to support regulatory journeys.
What types of studies does bioaccess® manage?
Bioaccess® manages various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What are the benefits of engaging in the FDA pre-submission process?
Engaging in the FDA pre-submission process can significantly expedite the path to market authorization, as approximately two-thirds of requests involve discussions with the FDA, which can establish a strong foundation for successful submissions.
Are there any fees associated with the FDA pre-submission process?
There are no user fees associated with pre-submissions, making it a cost-effective strategy for sponsors.