


The article emphasizes the critical role of mastering the FDA Product Code Database in ensuring the success of medical devices. By facilitating accurate classification and compliance with regulatory standards, understanding the database's structure and effectively navigating it can significantly influence product categorization. This expertise streamlines approval processes and enhances market entry success, as exemplified by the case of the iluminage Skin Smoothing Laser.
Navigating the complex landscape of medical device regulation can be daunting. However, the FDA Product Code Database stands as a vital tool for manufacturers and innovators alike. This comprehensive resource classifies medical equipment based on intended use and technological features, directly influencing compliance pathways and market entry strategies.
With the stakes so high, how can one ensure they are leveraging this database effectively to avoid costly misclassifications and delays? Understanding the intricacies of the FDA Product Code Database is essential for anyone aiming to succeed in the competitive medical device market.
The fda product code database serves as a crucial resource for classifying medical equipment according to their intended use and technological features. Each product code distinctly identifies a specific type of equipment, facilitating a streamlined compliance process. For producers and investigators, a comprehensive understanding of this database is essential, as it directly impacts the categorization of their equipment and the associated compliance requirements.
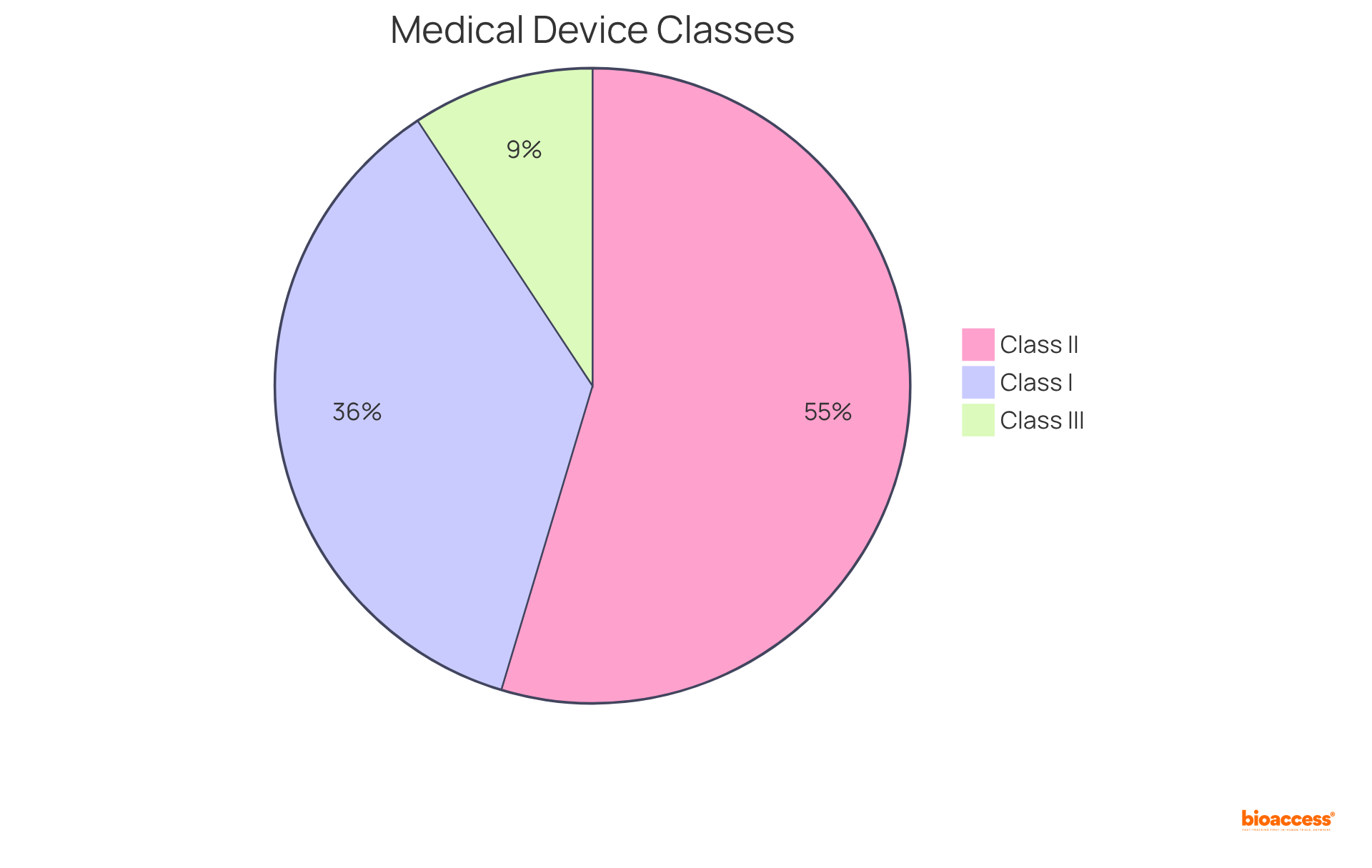
In 2020,
This categorization system underscores the importance of accurately identifying an object's type to ensure adherence to relevant regulations.
To navigate the fda product code database effectively, users should familiarize themselves with its structure, including the assignment of product codes and their implications for product categorization. Manufacturers can enhance their compliance success rates by utilizing the fda product code database, as illustrated by the case of the iluminage Skin Smoothing Laser. This FDA-approved device, designed for home use, successfully entered the market by aligning its categorization with compliance requirements, showcasing the vital role of understanding product codes in achieving adherence. The product code assigned to the Skin Smoothing Laser was instrumental in defining its category and ensuring it met the necessary regulatory standards for market entry.
Regulatory specialists emphasize that a clear understanding of product codes significantly influences equipment categorization, ultimately resulting in more efficient approval procedures. As the landscape of medical instruments continues to evolve, effectively utilizing the fda product code database will remain crucial for manufacturers seeking to introduce innovative solutions to the market.
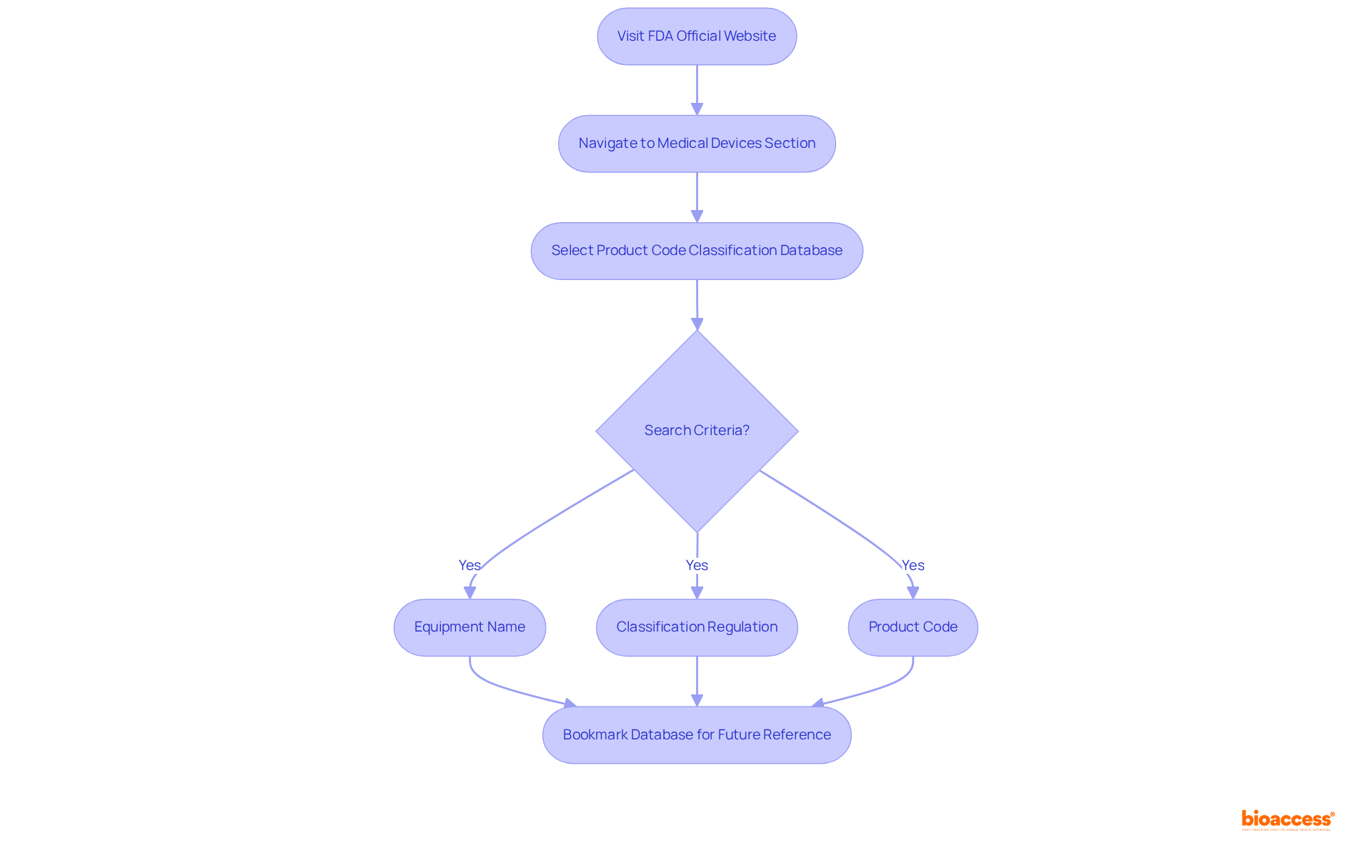
Accessing the FDA product code database is not only straightforward but also essential for innovators in Medtech, Biopharma, and Radiopharma. Start by visiting the FDA's official website and navigating to the 'Medical Devices' section. From there, select 'Product Code Classification Database.' This resource allows you to search for product codes using various criteria, including:
To enhance your search efficiency, familiarize yourself with the available functionalities, such as keyword searches and filters. For ongoing reference, consider bookmarking the database, as it will serve as an invaluable tool throughout your product development journey.
Understanding how to effectively navigate this database is crucial, particularly for startups aiming to accelerate their clinical studies. For instance, bioaccess® has successfully utilized this database to simplify the compliance process, leveraging Latin America's oversight efficiency and the diverse patient groups in the Balkans to secure quicker ethical approvals. By mastering the FDA product code database, you can position your products for success in these dynamic markets. Moreover, consider the importance of effective navigation, such as identifying the correct product code for a new medical instrument, which significantly influences your regulatory strategy and market entry timeline.
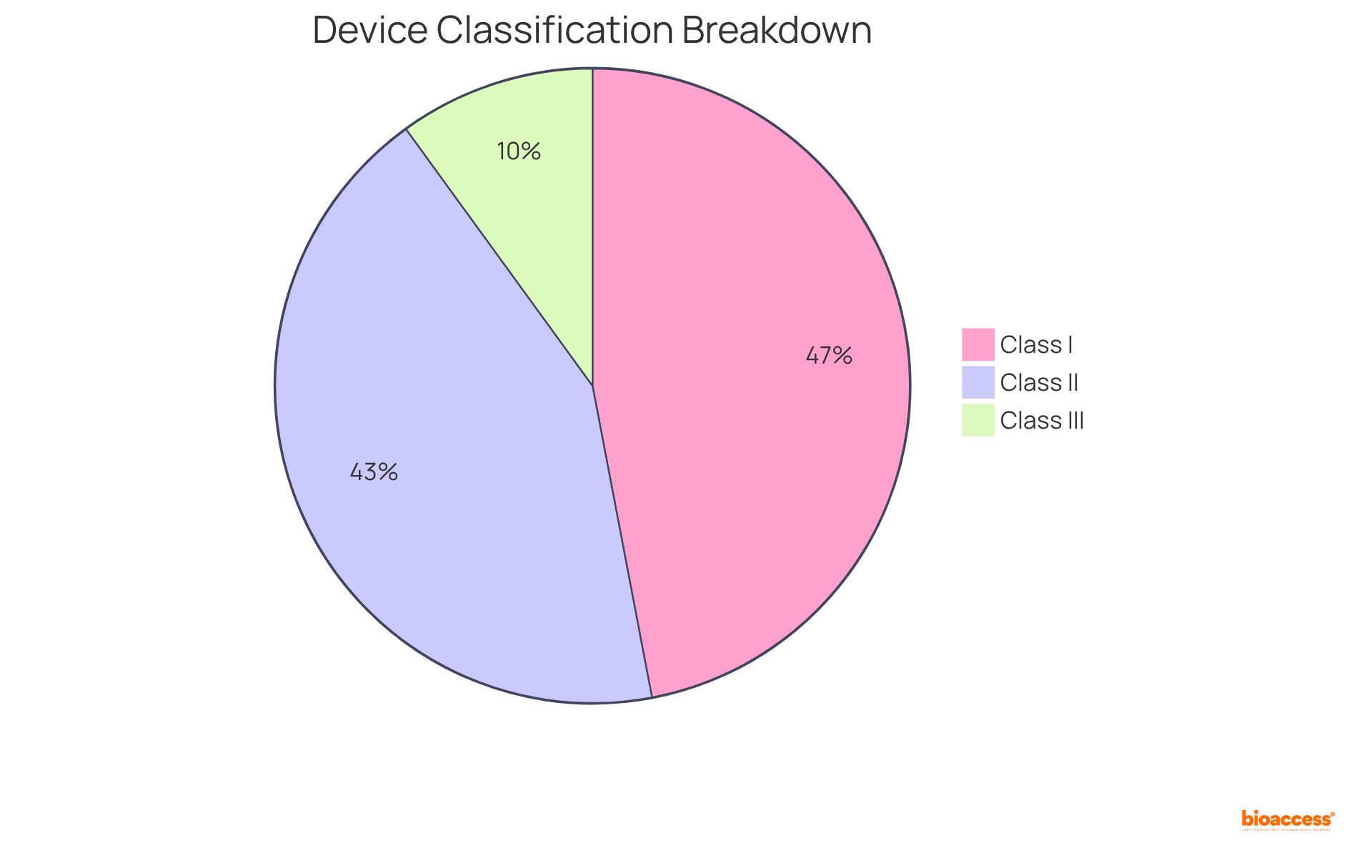
To effectively search for product codes, start by entering relevant keywords related to your equipment in the FDA product code database's search bar. Once you locate the product code, pay close attention to its description, classification, and associated regulations. Understanding the classification—Class I, II, or III—is essential, as it dictates the compliance route your equipment must follow.
Recent data indicates that approximately:
reflecting the varying levels of risk and oversight requirements. Notably, the FDA reports an average of 1.5 recalls per week, underscoring the importance of understanding product categories in the context of oversight and potential recalls. Furthermore, there was a 64.76% increase in unit recalls from 2016 to 2017, highlighting the growing importance of compliance and the necessity of understanding categories within the current regulatory landscape.
Producers of Class I products must also implement quality systems in accordance with Good Manufacturing Practices (GMP) standards. Familiarizing yourself with the terminology used in the FDA product code database will further enhance your understanding of the categorization process, empowering you to make informed decisions regarding your product development and market introduction.
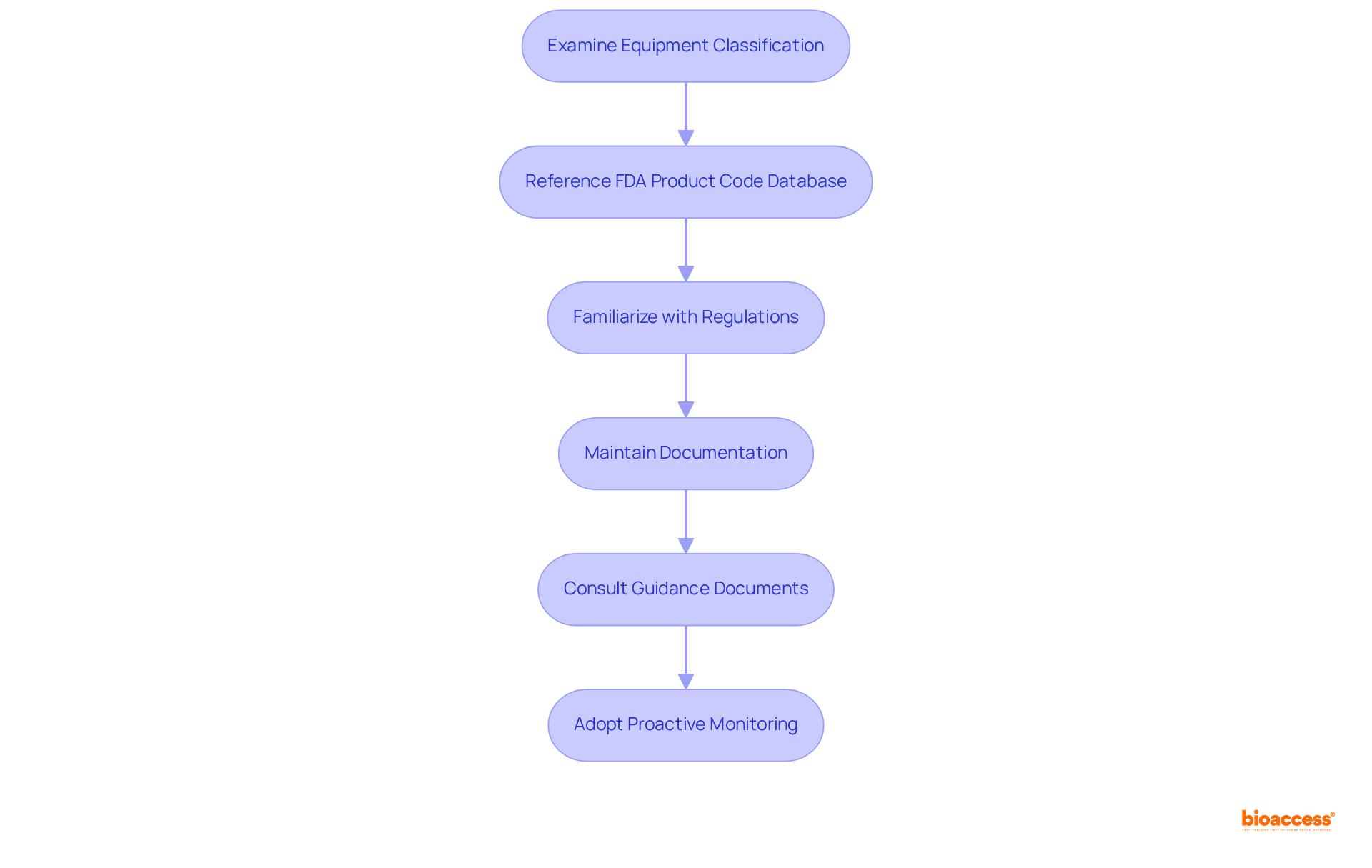
To ensure adherence to regulatory requirements, begin by thoroughly examining your equipment's classification and referencing the FDA product code database. Familiarize yourself with the specific regulations pertinent to your equipment category, including:
For example, Class III products necessitate a Premarket Approval (PMA) application, which represents the most stringent type of marketing application mandated by the FDA. The FDA's review of a PMA application typically spans 274 days, making it essential to plan accordingly.
Maintain meticulous documentation of your development process, encompassing:
Regularly consult the FDA's guidance documents and updates to remain informed about any regulatory changes that may affect your device. Collaborating with compliance consultants can provide valuable insights into navigating complex adherence challenges, as they often emphasize that "compliance is not merely an obstacle to overcome; it’s a continual dedication to patient safety and product quality."
Furthermore, adopting a proactive approach to oversight monitoring, such as subscribing to updates from the FDA, can assist manufacturers in staying ahead of compliance requirements. By integrating these strategies, manufacturers can enhance their likelihood of successful market entry while maintaining compliance in a dynamic oversight environment.
Misclassification of medical products frequently stems from misunderstandings of product codes, improper categorization, or ambiguous regulatory requirements. Statistics reveal that a significant percentage of medical equipment is misclassified, leading to delays in the approval process.
To effectively troubleshoot these categorization challenges, consider the following steps:
This proactive approach not only assists in resolving current classification problems but also aids in preventing similar issues in the future. Additionally, understanding the FDA's Intercenter Agreements can provide further context on the regulatory landscape, which is pertinent to the troubleshooting process.
Mastering the FDA Product Code Database is essential for successfully navigating the medical device landscape. This resource not only aids in the classification of medical equipment but also directly influences compliance and regulatory processes. Understanding the nuances of product codes can streamline the path to market, ensuring that manufacturers meet the necessary standards for different classes of medical devices.
Key insights from the article emphasize the importance of accurately categorizing devices based on their intended use and technological features. The classification system—divided into Class I, II, and III—demonstrates the varying levels of oversight each category requires. By effectively utilizing the FDA product code database, manufacturers can avoid common pitfalls associated with misclassification and enhance their chances of timely market entry. The case studies presented, such as the ilumage Skin Smoothing Laser, exemplify how a thorough understanding of product codes can lead to successful compliance and market strategies.
Ultimately, the significance of the FDA Product Code Database extends beyond mere compliance; it represents a commitment to innovation and patient safety in the medical device industry. As the regulatory landscape continues to evolve, manufacturers are encouraged to adopt a proactive approach to navigating this resource. By doing so, they not only position themselves for success but also contribute to the overall advancement of healthcare solutions. Embracing this knowledge and applying it effectively can lead to groundbreaking developments in medical technology.
What is the FDA product code database?
The FDA product code database is a resource used for classifying medical equipment based on their intended use and technological features. It provides distinct product codes that identify specific types of equipment, aiding in the compliance process.
Why is understanding the FDA product code database important for manufacturers?
A comprehensive understanding of the FDA product code database is essential for manufacturers and investigators as it directly impacts the categorization of their equipment and the associated compliance requirements.
What are the classifications of medical instruments according to the FDA?
In 2020, 35% of controlled medical instruments were classified as Class I (minimal oversight), 53% as Class II (stricter supervision), and 9% as Class III (highest risk with rigorous regulatory standards).
How can the FDA product code database aid in compliance?
By utilizing the FDA product code database, manufacturers can enhance their compliance success rates, as it helps in accurately identifying an object's type and ensuring adherence to relevant regulations.
How can users access the FDA product code database?
Users can access the FDA product code database by visiting the FDA's official website, navigating to the 'Medical Devices' section, and selecting 'Product Code Classification Database.'
What search criteria can be used in the FDA product code database?
Users can search for product codes using various criteria, including equipment name, classification regulation, and the product code itself.
What tips can improve the efficiency of searching the FDA product code database?
Familiarizing oneself with functionalities such as keyword searches and filters can enhance search efficiency. Bookmarking the database is also recommended for ongoing reference.
How has the FDA product code database benefited companies in the Medtech field?
Companies like bioaccess® have utilized the database to simplify compliance processes and secure quicker ethical approvals, positioning their products for success in dynamic markets.
Why is effective navigation of the FDA product code database crucial for startups?
Effective navigation is crucial for startups as it significantly influences their regulatory strategy and market entry timeline, particularly when identifying the correct product code for new medical instruments.