


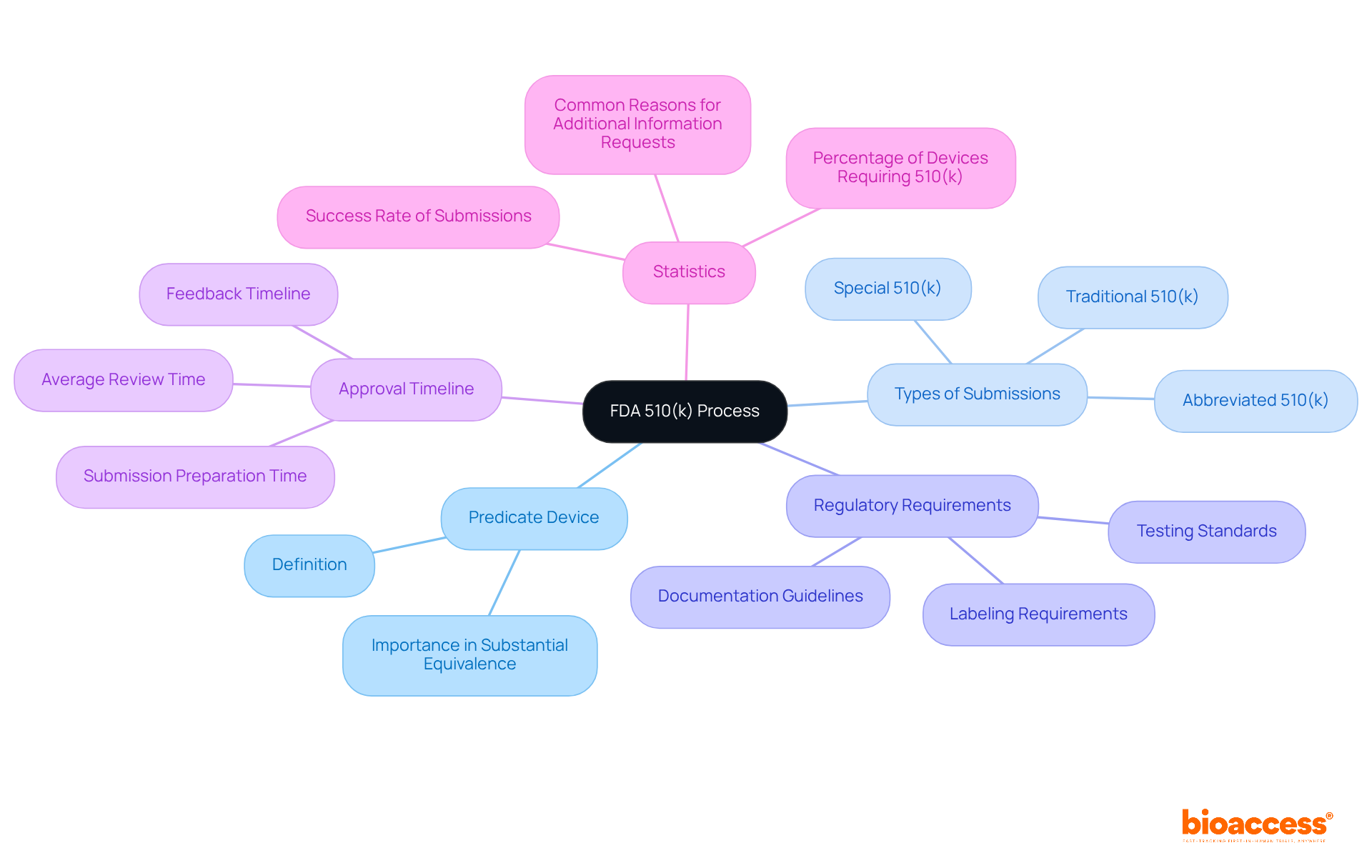
This article offers comprehensive, step-by-step guidance on mastering the FDA 510(k) process, a critical requirement for medical equipment producers to demonstrate the safety and effectiveness of their products. It meticulously details essential components of the process, including:
The emphasis on a thorough grasp of these elements significantly enhances the chances of successful market entry, underscoring the importance of expertise in navigating this complex landscape.
Navigating the FDA 510(k) process resembles traversing a complex maze, particularly as the landscape of medical device regulation continues to evolve. Projections indicate that a staggering 82% of medical devices will require a 510(k) application by 2025; thus, understanding this essential pathway is critical for manufacturers. This guide provides a step-by-step approach to mastering the FDA search 510(k) process, equipping readers with the necessary tools to enhance their chances of successful approval.
However, what occurs when the search yields no results or becomes overwhelming? By exploring these challenges and solutions, industry professionals will be empowered to tackle the intricacies of FDA compliance effectively.
The fda search 510k process serves as a crucial premarket application route for medical equipment producers, enabling them to demonstrate that their product is safe, effective, and substantially equivalent to an already marketed item. Understanding this process requires knowledge of the categories of equipment that necessitate a 510(k) filing, the standards for determining substantial equivalence, and the comprehensive approval timeline. Key components include:
In 2025, approximately 82% of medical devices entering the market will require a 510(k) application, highlighting the importance of mastering this process. Regulatory experts assert that a thorough understanding of the fda search 510k process is vital for successful market entry. For instance, one expert noted that navigating the complexities of medical product approvals requires a clear understanding of the differences between 510(k) clearance and PMA processes. By mastering these elements, you will be better equipped to navigate the complexities of the 510(k) application process, ultimately enhancing your chances of successful approval.
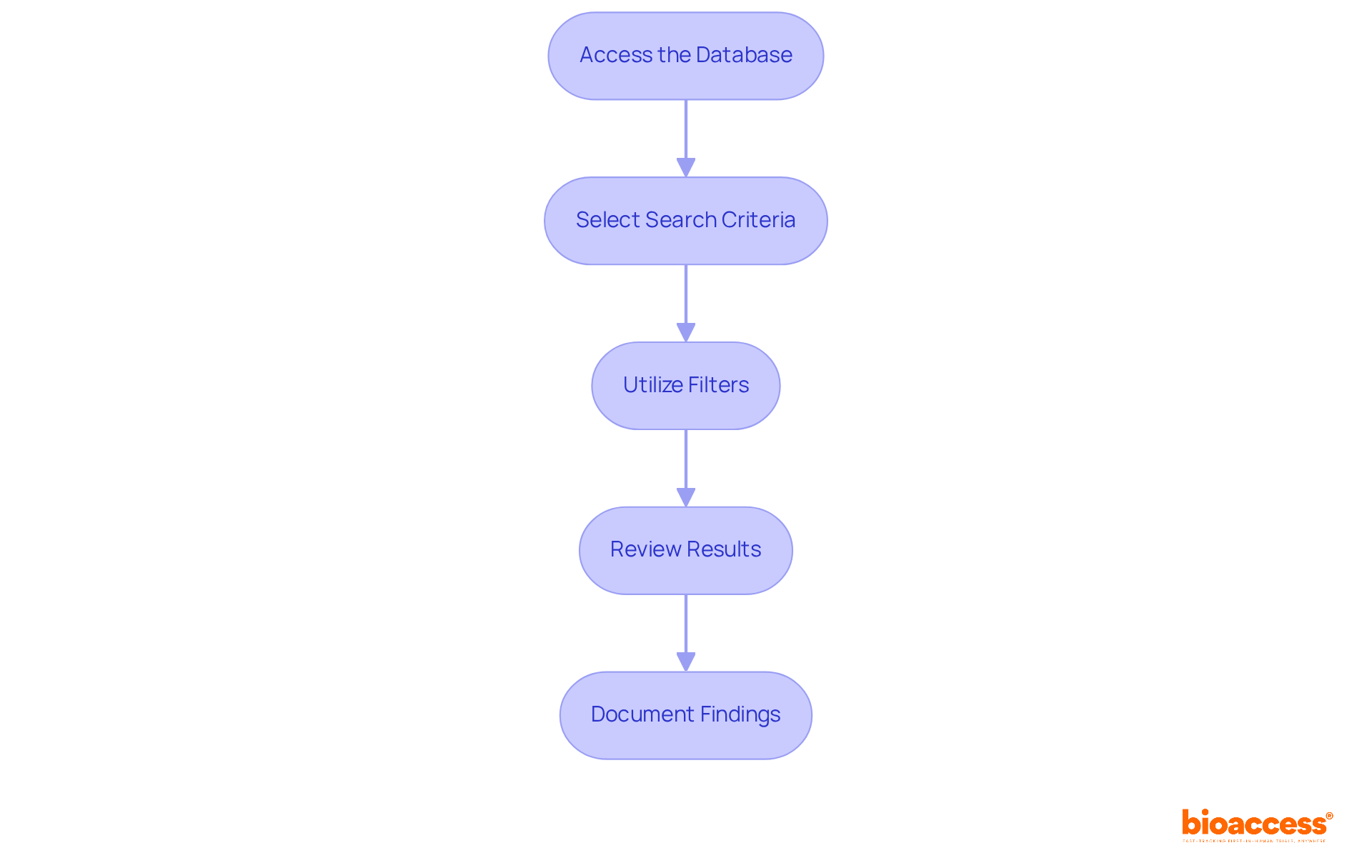
To effectively search the FDA 510(k) database, it is essential to follow these structured steps:
By adhering to these steps, you can efficiently locate the necessary information for your FDA search 510(k) application. Be prepared for common challenges in navigating the database, as many submissions may require additional information requests, which can lead to delays.
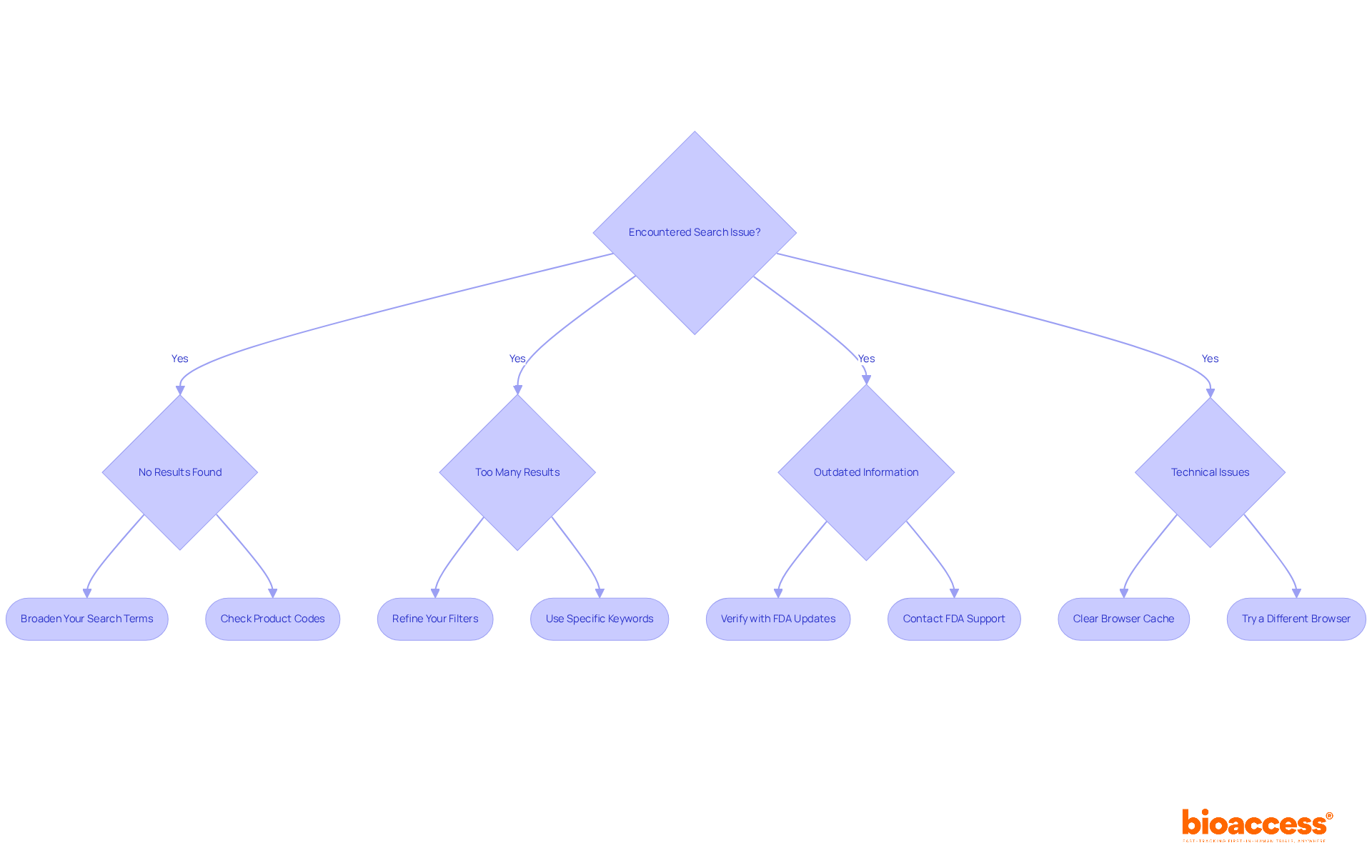
While performing an FDA search 510k, you may encounter several common issues. Understanding how to troubleshoot them effectively is essential for navigating this critical resource:
No Results Found: If your search yields no results, consider the following strategies:
Too Many Results: If your search returns an overwhelming number of results, try these approaches:
Outdated Information: If you suspect the information is outdated, it is crucial to:
Technical Issues: If you experience technical difficulties during your search, you can:
By being proactive and prepared for these challenges, you can enhance your efficiency in navigating the FDA search 510k database. As noted by industry experts, addressing these common issues can significantly streamline the search process and improve outcomes.
Mastering the FDA 510(k) process is essential for medical device manufacturers aiming for successful market entry. This guide highlights the critical steps and insights necessary to navigate the complexities of the 510(k) application, emphasizing the importance of understanding predicate devices, submission types, and regulatory requirements. By grasping these elements, producers can effectively demonstrate their products' safety and efficacy, ensuring compliance with FDA standards.
Key arguments presented include:
Following the outlined steps for database navigation not only enhances search efficiency but also aids in identifying relevant predicate devices, which are pivotal for demonstrating substantial equivalence. Additionally, being prepared for potential challenges can significantly improve the likelihood of successful approval.
Ultimately, the FDA 510(k) process is a vital pathway for medical devices entering the market. By employing best practices in searching and understanding the requirements, manufacturers can streamline their application process and contribute to the safety and effectiveness of medical innovations. Embracing this knowledge is crucial for anyone involved in the medical device industry, as it directly impacts product success and patient safety.
What is the FDA 510(k) process?
The FDA 510(k) process is a premarket application route for medical equipment producers to demonstrate that their product is safe, effective, and substantially equivalent to an already marketed item.
What is a predicate device?
A predicate device is an already legally marketed device that serves as a benchmark for demonstrating substantial equivalence in the 510(k) process.
What types of 510(k) submissions are there?
There are three types of 510(k) submissions: Traditional, Abbreviated, and Special 510(k)s, each with distinct requirements tailored to different scenarios.
Why is it important to understand the FDA's regulatory requirements?
Understanding the FDA's regulatory requirements regarding documentation, testing, and labeling is essential to ensure compliance and enhance the chances of successful approval for medical devices.
What percentage of medical devices will require a 510(k) application by 2025?
Approximately 82% of medical devices entering the market will require a 510(k) application by 2025.
How does the 510(k) process differ from the PMA process?
The 510(k) process is focused on demonstrating substantial equivalence to a predicate device, while the PMA (Premarket Approval) process is typically more rigorous and is used for devices that are considered high-risk and require more extensive evidence of safety and effectiveness.