


The article outlines the crucial steps necessary for effectively navigating the medical device regulatory calendar in Latin America (LATAM). It underscores the significance of comprehending the diverse oversight bodies, the classification of equipment, documentation requirements, and ongoing compliance strategies. Each of these elements is vital for facilitating a seamless market entry and obtaining regulatory approval for medical devices in this rapidly expanding region.
Navigating the complex landscape of medical device regulation in Latin America presents both challenges and opportunities for manufacturers eager to enter this burgeoning market. With the region's medical equipment sector expected to grow significantly, understanding the intricate regulatory calendar is crucial for success.
How can companies effectively prepare for submissions, manage compliance, and stay ahead of evolving regulations amidst the diverse requirements of countries like Brazil, Mexico, and Colombia?
This article delves into the key steps necessary for mastering the LATAM medical device regulatory calendar, ensuring a smoother path to market entry and sustained compliance.
To effectively navigate the medical device regulatory calendar LATAM, understanding several key aspects is essential.
Oversight Bodies: Acquaint yourself with the primary oversight organizations in each nation, including ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia. Each authority operates under distinct guidelines and approval processes, which can significantly impact your product's market entry.
Classification of Equipment: Medical instruments in LATAM are categorized based on risk levels (Class I, II, III), determining the compliance pathway and requirements. Understanding this classification is crucial for determining the necessary steps for approval.
Documentation Requirements: Each governing body mandates specific documentation for device approval, including technical files, clinical data, and labeling information. A thorough comprehension of these requirements is vital to ensure compliance and expedite the approval process.
Market Trends: Staying updated on the latest trends and regulatory changes is imperative, as these can influence your strategy. For instance, Brazil's recent updates aim to simplify the approval process, potentially decreasing timelines for entry. The Latin American medical equipment sector is anticipated to expand from USD 7.50 billion in 2024 to USD 14.15 billion by 2033, emphasizing the importance of managing these regulations efficiently.
Cultural Considerations: Recognize the cultural and operational variances among Latin American nations, as these can influence compliance interactions and entry approaches. Tailoring your approach to each country's unique environment can enhance your chances of success.
Competitive Landscape: Be aware of the competitive dynamics in the Latin American region, where high equipment costs and compliance challenges pose significant barriers. Understanding these challenges will help you devise strategies to overcome them.
By mastering these foundational elements, you will be well-prepared to navigate the medical device regulatory calendar LATAM, which will facilitate a smoother path to market for your medical devices.
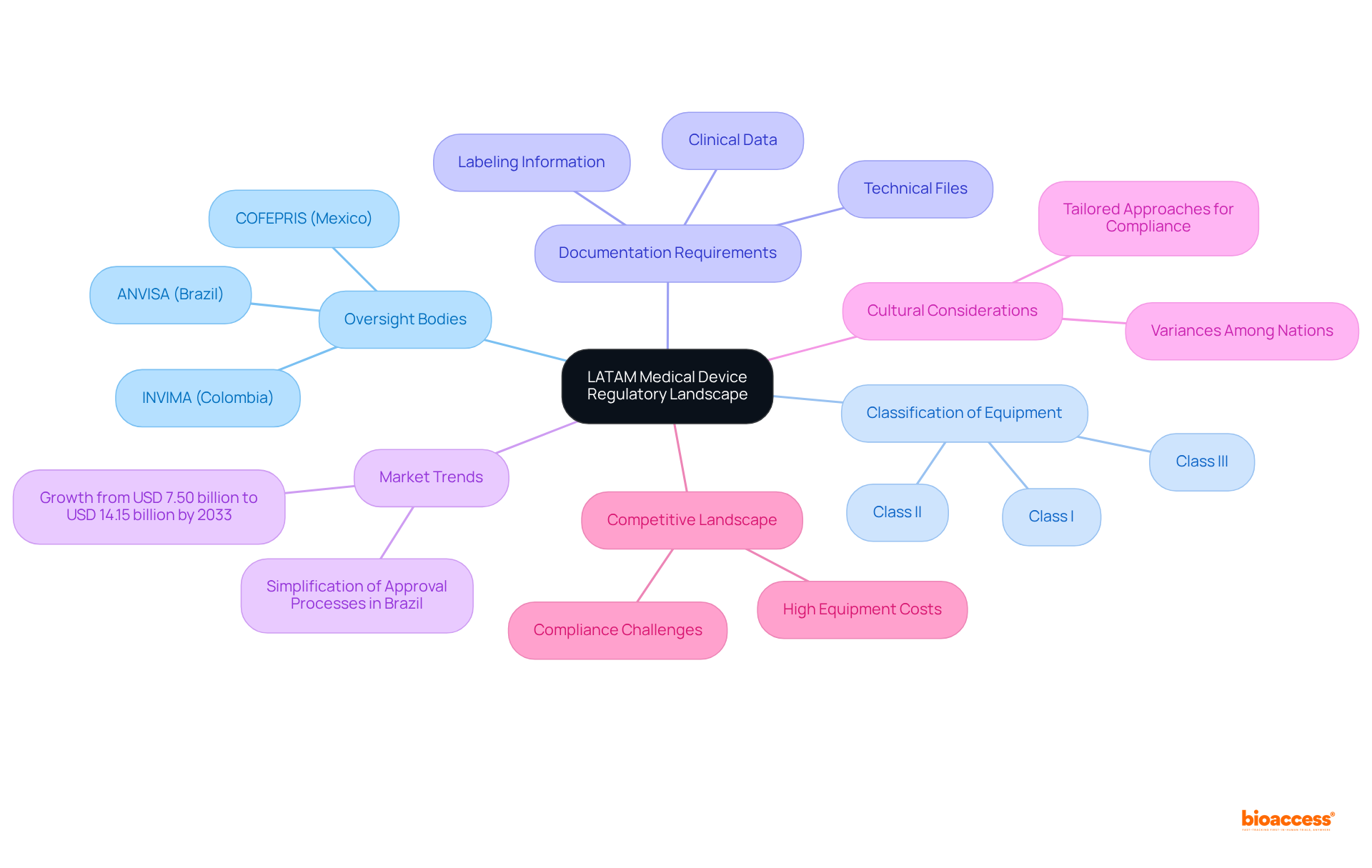
To effectively prepare for regulatory submissions in LATAM, it is crucial to follow these essential steps:
Perform a Compliance Gap Analysis: Assess your current documentation and procedures against the requirements established by the target oversight authority. This analysis will identify any compliance gaps that must be addressed, ensuring alignment with local regulations.
Compile Technical Documentation: Assemble all necessary technical documentation, including detailed product specifications, manufacturing processes, and quality management system information. It is vital that these documents are current and comply with local legal standards.
Engage Local Experts: Collaborate with local compliance consultants who possess in-depth knowledge of the LATAM market. As Katherine Ruiz, an expert in Affairs for Medical Devices and In Vitro Diagnostics, states, "Manufacturers need to conduct comprehensive research on the unique requirements of each jurisdiction and utilize the knowledge of local compliance professionals." Their expertise is invaluable in navigating complex regulatory requirements and enhancing the quality of your submissions.
Prepare Clinical Data: If relevant, gather clinical data that substantiates the safety and efficacy of your device. This may include results from clinical trials conducted within LATAM or other regions, which are critical for demonstrating compliance.
Draft Submission Dossier: Create a comprehensive submission dossier that includes all required documents, forms, and supporting materials. Ensure that the dossier is well-organized and clearly labeled to facilitate a smooth review process by regulatory authorities. The structured process for medical device registration in Brazil under ANVISA, for example, highlights the importance of thorough documentation and adherence to local standards as outlined in the medical device regulatory calendar latam.
Review and Revise: Prior to submission, conduct a meticulous review of the dossier to confirm its accuracy and completeness. Revise any sections that require clarification or additional information to avoid delays in the approval process.
By adhering to these steps, you can significantly enhance the likelihood of a smooth approval process, ultimately accelerating your product's entry into the Latin American region. Additionally, leveraging bioaccess®'s market access services can further facilitate the successful commercialization of your innovative products in this multi-billion dollar healthcare market.
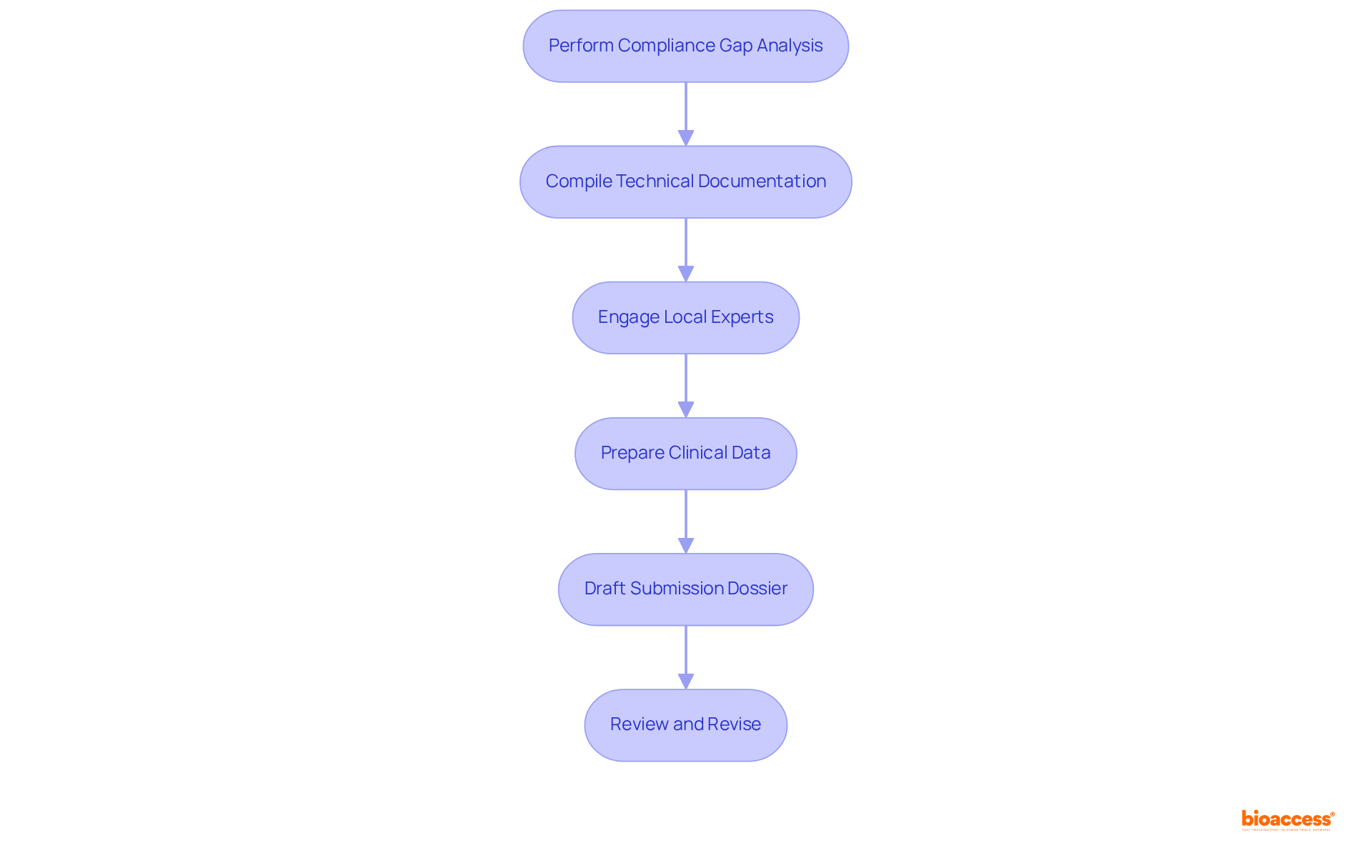
To manage timelines and deadlines effectively in the LATAM regulatory process, consider the following strategies:
By applying these strategies, you can effectively manage the medical device regulatory calendar LATAM and ensure prompt submissions.
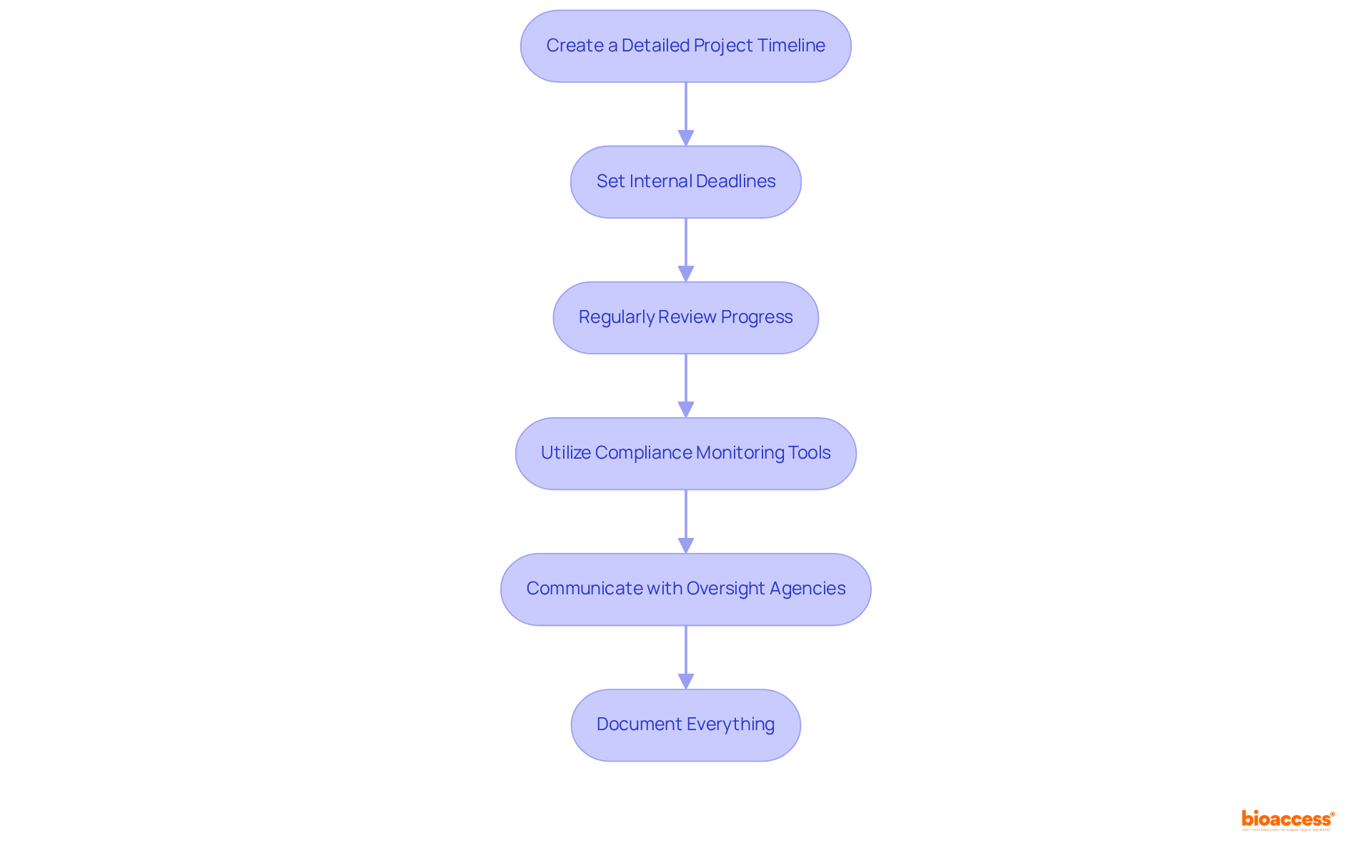
To ensure ongoing compliance and monitoring in the LATAM medical device market, it is imperative to follow these essential steps:
Establish a Compliance Management System: Implement a robust compliance management system that encompasses policies, procedures, and training for your team. This system must address all aspects of compliance with regulations, including post-market surveillance and reporting, which are essential in a rapidly evolving market.
Monitor Compliance Updates: Stay informed about compliance changes that may affect your products. Engaging with local compliance specialists, subscribing to industry newsletters, and attending compliance conferences can provide valuable insights into the evolving landscape. With the medical device regulatory calendar LATAM indicating that the medical equipment compliance market in Latin America is projected to reach USD 446.2 million by 2030, expanding at a CAGR of 9.4% from 2025 to 2030, remaining informed is crucial for ensuring adherence.
Conduct Regular Audits: Schedule regular internal audits to evaluate compliance with regulatory requirements. These audits help identify areas for enhancement and ensure that your procedures align with current regulations, which have seen a 15% rise in approvals recently, with 98 items obtaining clearance. The healthcare equipment market in Latin America is anticipated to generate revenue of USD 37.23 billion by 2025, underscoring the importance of adherence to the medical device regulatory calendar latam in this rapidly growing market.
Engage with Stakeholders: Cultivate strong relationships with key stakeholders, including governing bodies, healthcare providers, and industry associations. These connections can offer valuable insights and support for compliance efforts, especially considering the medical device regulatory calendar latam, as the demand for affordable medical devices increases in LATAM due to a large uninsured population.
Implement a Reporting System: Develop a system for reporting adverse events and product issues to governing bodies as necessary. Ensure that your team is well-trained on the reporting process and understands the significance of timely communication, particularly in a market where the prevalence of chronic diseases is driving demand for effective medical solutions.
Review and Update Documentation: Regularly review and update all regulatory documentation, including labeling, technical files, and quality management system records. Accurate documentation is essential, particularly as customer preferences in Latin America increasingly favor technologically advanced and user-friendly medical devices.
By prioritizing ongoing compliance and monitoring according to the medical device regulatory calendar latam, you can safeguard your products and maintain a strong presence in the LATAM market, which is expected to grow at a CAGR of 5.87% from 2025 to 2030.
Navigating the medical device regulatory landscape in LATAM presents a multifaceted endeavor, necessitating a comprehensive understanding of diverse national regulations, classification systems, and documentation requirements. By mastering these foundational elements, stakeholders significantly enhance their chances of successfully bringing medical devices to market while adhering to local compliance standards.
The article outlines key strategies that emphasize the importance of thorough preparation for regulatory submissions, effective timeline management, and ongoing compliance monitoring. Conducting compliance gap analyses, engaging local experts, and maintaining open communication with oversight agencies are vital steps that streamline the approval process. Furthermore, staying informed about market trends and regulatory updates is crucial for adapting to the evolving landscape of the LATAM medical device sector.
Ultimately, mastering the medical device regulatory calendar in LATAM transcends mere deadline management; it embodies a proactive approach to compliance that ensures product safety and efficacy in a burgeoning market. As the demand for innovative medical solutions continues to rise, embracing these strategies empowers manufacturers to effectively navigate challenges and capitalize on the immense opportunities within the LATAM healthcare market.
What are the primary oversight bodies for medical devices in LATAM?
The primary oversight bodies include ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia. Each operates under distinct guidelines and approval processes.
How are medical devices classified in LATAM?
Medical devices in LATAM are classified based on risk levels into three categories: Class I, II, and III. This classification determines the compliance pathway and requirements for approval.
What documentation is required for medical device approval in LATAM?
Each governing body mandates specific documentation, including technical files, clinical data, and labeling information, which are essential for compliance and expediting the approval process.
Why is it important to stay updated on market trends in LATAM?
Staying updated on market trends and regulatory changes is crucial as they can influence your strategy. For example, recent updates in Brazil aim to simplify the approval process, potentially reducing entry timelines.
What is the projected growth of the Latin American medical equipment sector?
The Latin American medical equipment sector is anticipated to grow from USD 7.50 billion in 2024 to USD 14.15 billion by 2033, highlighting the importance of managing regulations effectively.
How do cultural considerations affect compliance in LATAM?
Cultural and operational variances among Latin American nations can influence compliance interactions and entry approaches. Tailoring your approach to each country's unique environment can enhance success.
What are the competitive challenges in the LATAM medical device market?
High equipment costs and compliance challenges pose significant barriers in the Latin American region. Understanding these challenges is essential for devising effective strategies to overcome them.