


The Mexico English IFU Acceptance Policy stipulates that all medical products sold in Mexico must provide clear Instructions for Use in both Spanish and English, ensuring compliance with local regulations. This policy is crucial for enhancing user safety and improving market performance, particularly for companies targeting English-speaking consumers in Mexico.
Accurate content, cultural relevance, and regulatory approval are not just recommendations; they are essential components of successful market entry. By adhering to these guidelines, companies can significantly bolster their credibility and operational effectiveness in the Medtech landscape.
Navigating the complexities of medical device regulations in Mexico presents a significant challenge, particularly in light of the recent emphasis on the Mexico English IFU Acceptance Policy. This policy mandates that all medical products sold within the country include clear and compliant Instructions for Use, specifically catering to English-speaking communities and international firms.
As the demand for accessible medical information continues to rise, organizations face the critical task of ensuring that these instructions are not only accurately translated but also culturally relevant and user-friendly.
How can organizations effectively meet these stringent requirements while simultaneously enhancing user safety and achieving market success?
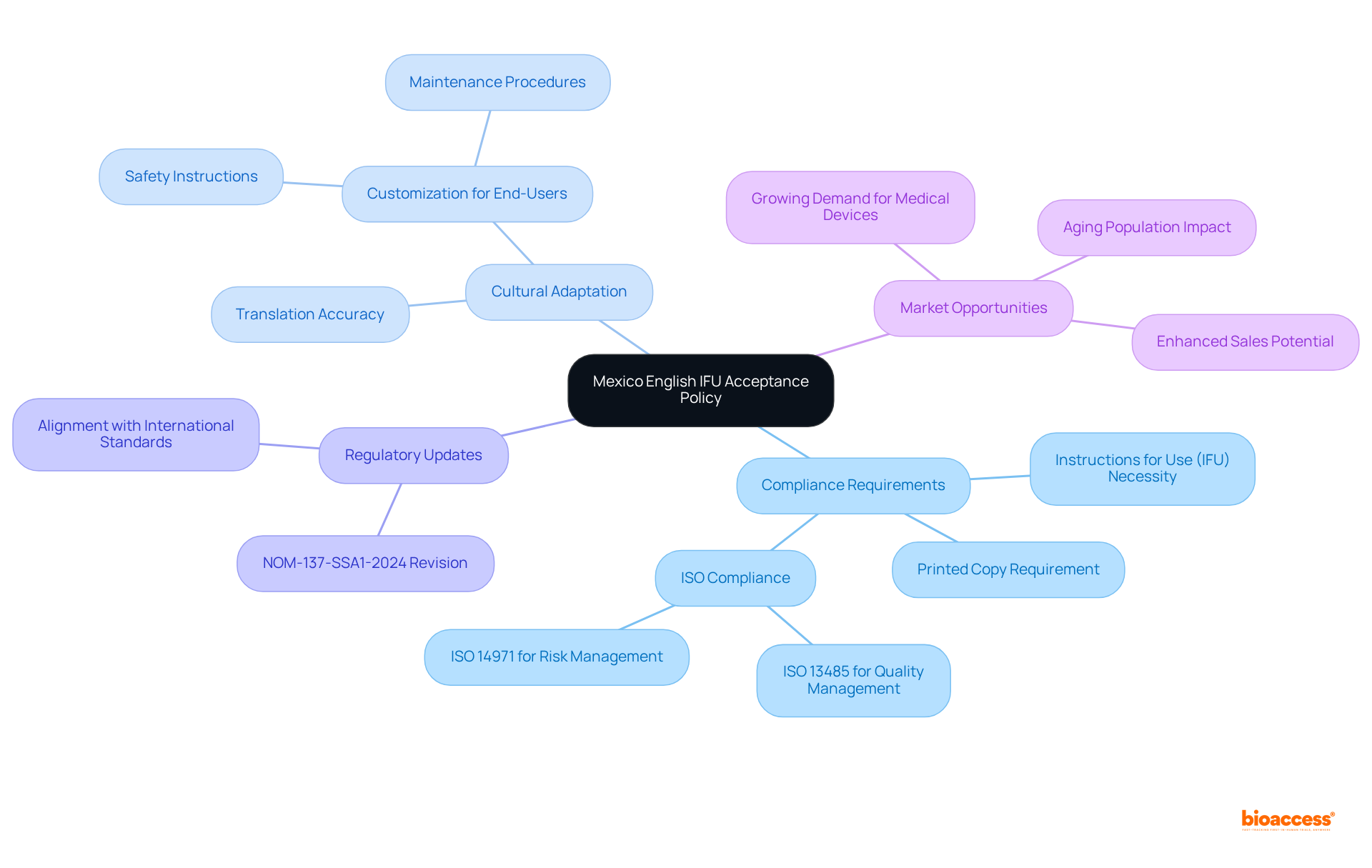
The Mexico English IFU Acceptance Policy requires that all medical products sold in Mexico must include clear Instructions for Use that comply with local regulations. This policy, known as the Mexico English IFU acceptance policy, is particularly relevant for products targeted at English-speaking communities and global firms seeking to enter the Mexican market. While Spanish serves as the primary language for labeling and instructions, versions in other languages are permissible under specific conditions. It is crucial to ensure that these documents are not only translated accurately but also customized to meet the cultural and practical needs of end-users. This includes providing clear instructions on equipment operation, safety precautions, and maintenance procedures—elements that are vital for user safety and equipment efficacy. Additionally, a printed copy of the IFU is required to satisfy regulatory compliance.
Recent updates to medical equipment labeling regulations, particularly the draft revision of NOM-137-SSA1-2024, align with international standards and promote the Mexico English IFU acceptance policy. This change not only supports adherence but also broadens market entry opportunities for innovative medical products. Organizations that adeptly navigate these regulations can significantly enhance their sales in Mexico, particularly as the demand for devices catering to diverse linguistic needs continues to rise, driven by the aging population.
Integrating instructional materials effectively can enhance user experience and safety, ultimately leading to improved market performance. As the Mexican healthcare landscape evolves, staying abreast of regulatory changes is essential for successful market entry and sustained growth.
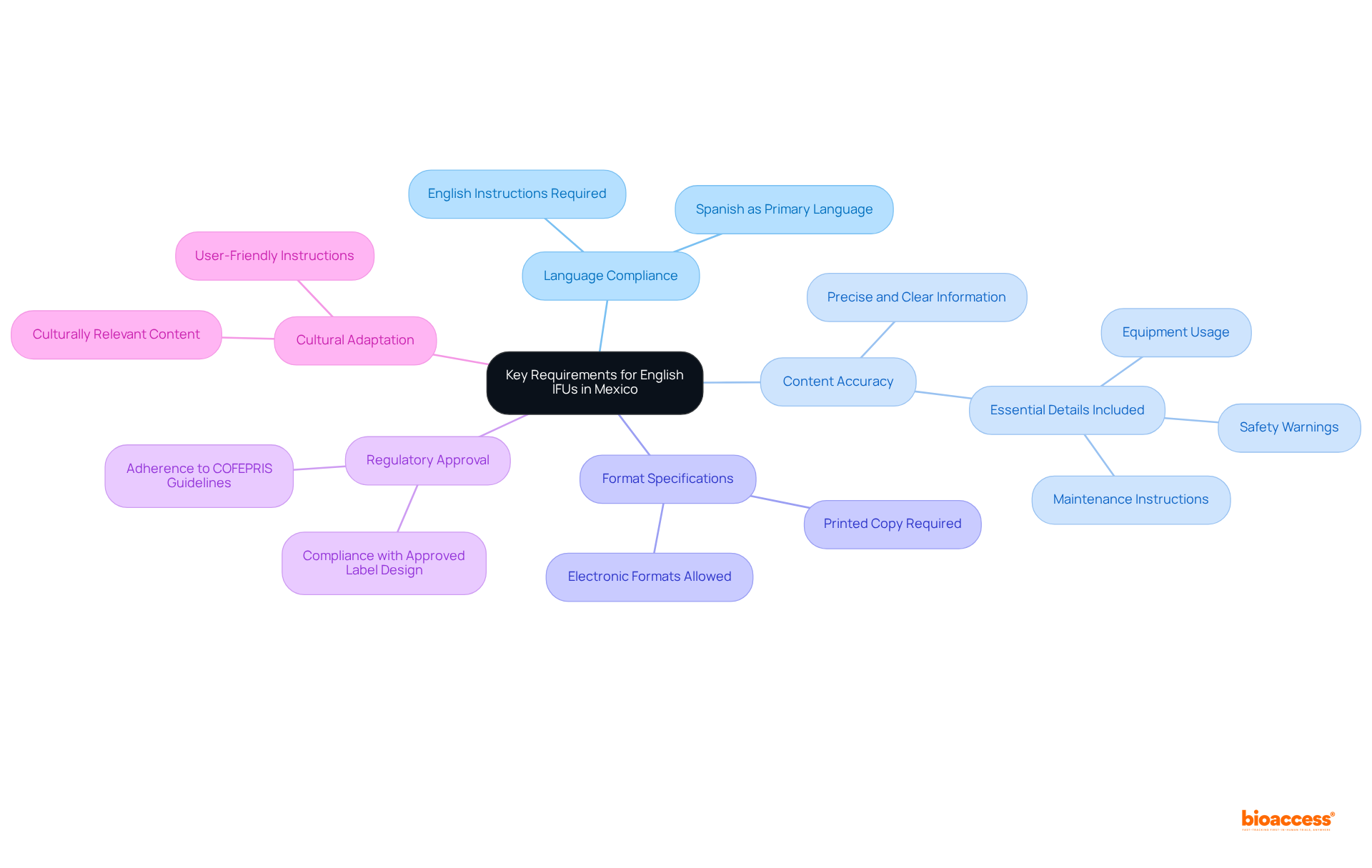
Key requirements for the Mexico English IFU acceptance policy are as follows:
Language Compliance: Instructions for Use must be provided in both Spanish and English, adhering to the Mexico English IFU acceptance policy, which stipulates Spanish as the primary language to meet regulatory standards.
Content Accuracy: The information presented must be precise, clear, and succinct, encompassing all essential details such as equipment usage, safety warnings, and maintenance instructions.
Format Specifications: Although instructions for use may be submitted in electronic formats, a printed copy is also necessary to ensure accessibility, in accordance with the recent draft revision of the labeling standard, NOM-137-SSA1-2024. Mexico English IFU acceptance policy, which permits electronic instructions in acceptable formats.
Regulatory Approval: All Instructions for Use must adhere to the guidelines established by COFEPRIS (Federal Commission for Protection against Sanitary Risks), which regulates medical devices in the country under the Mexico English IFU acceptance policy. It is imperative that printed labels correspond with the approved label design as part of compliance.
Cultural Adaptation: The content should be culturally relevant and easily comprehensible for the target audience, ensuring that the instructions are practical and user-friendly. This approach not only enhances compliance but also improves user experience and safety.
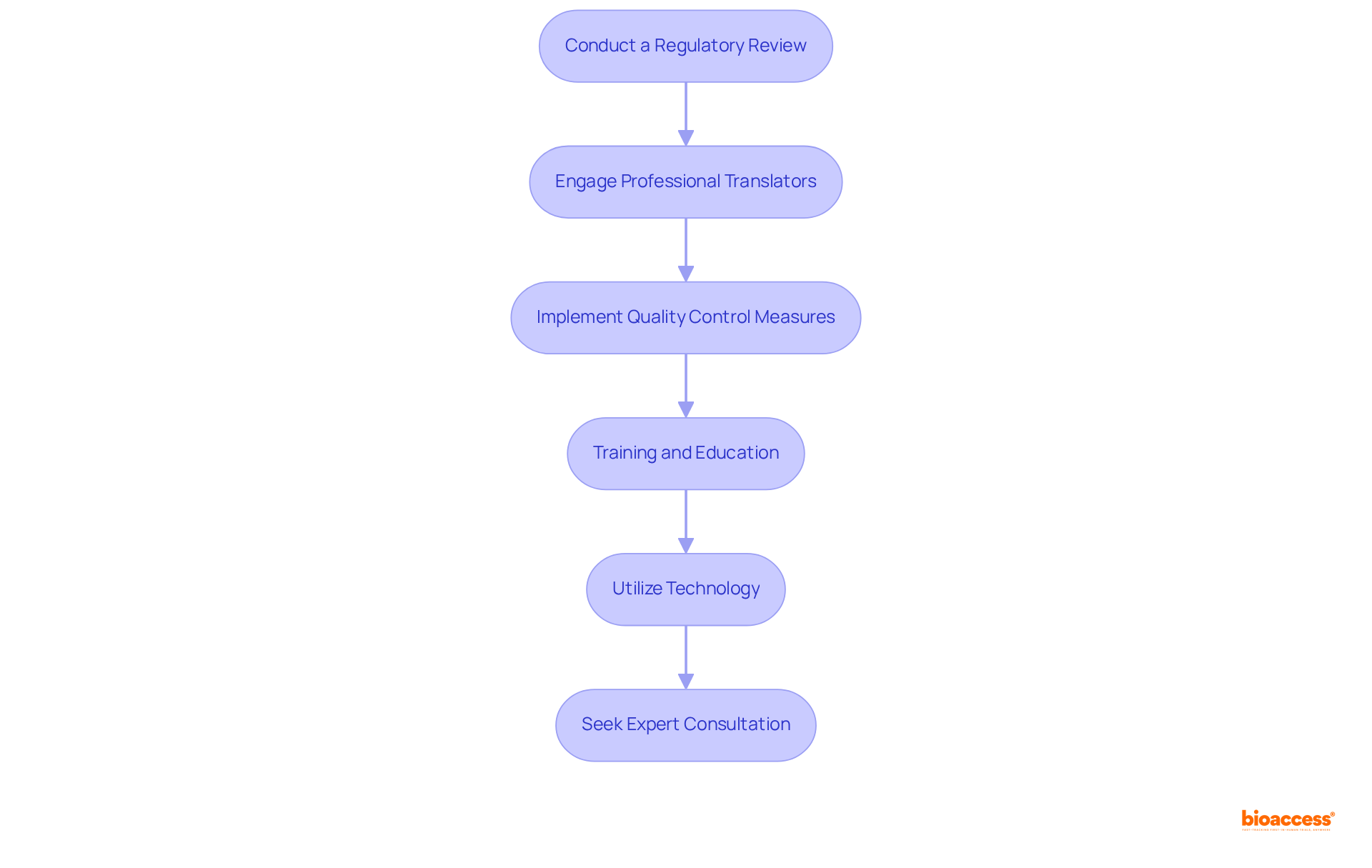
To ensure compliance with Mexico's English IFU Acceptance Policy, it is essential to implement the following strategies:
Understanding and adhering to the Mexico English IFU Acceptance Policy is crucial for any organization aiming to successfully market medical products in Mexico. This policy mandates the inclusion of clear and accurate Instructions for Use in both Spanish and English, while also emphasizing the importance of cultural relevance and user-friendliness in these documents. By aligning with these requirements, companies can enhance their compliance and ultimately improve their market presence.
The article outlines essential aspects of the policy, including the need for:
It highlights the significance of staying informed about regulatory changes, such as the recent draft revision of NOM-137-SSA1-2024. Engaging professional translators and implementing quality control measures are vital strategies for ensuring that the Instructions for Use meet both regulatory and user expectations.
In summary, navigating the complexities of the Mexico English IFU Acceptance Policy presents an opportunity for organizations to expand their reach in a growing market. By prioritizing compliance and user experience, companies not only adhere to regulations but also foster trust and safety among end-users. Embracing these practices is essential for sustainable growth and success in the Mexican healthcare landscape.
What is the Mexico English IFU Acceptance Policy?
The Mexico English IFU Acceptance Policy requires that all medical products sold in Mexico include clear Instructions for Use (IFU) that comply with local regulations, particularly for products aimed at English-speaking communities and global firms.
What language is primarily used for labeling and instructions in Mexico?
Spanish is the primary language for labeling and instructions in Mexico, but versions in other languages, such as English, are permissible under specific conditions.
What are the key requirements for the Instructions for Use (IFU)?
The IFU must be accurately translated and customized to meet the cultural and practical needs of end-users, including clear instructions on equipment operation, safety precautions, and maintenance procedures.
Is a printed copy of the IFU required?
Yes, a printed copy of the IFU is required to satisfy regulatory compliance.
How do recent updates to medical equipment labeling regulations affect the Mexico English IFU Acceptance Policy?
Recent updates, particularly the draft revision of NOM-137-SSA1-2024, align with international standards and promote the Mexico English IFU acceptance policy, broadening market entry opportunities for innovative medical products.
Why is it important for organizations to navigate these regulations effectively?
Organizations that effectively navigate these regulations can enhance their sales in Mexico, especially as the demand for devices catering to diverse linguistic needs increases due to the aging population.
How can integrating instructional materials impact user experience?
Effectively integrating instructional materials can enhance user experience and safety, ultimately leading to improved market performance in the evolving Mexican healthcare landscape.