


This article serves as a comprehensive guide to mastering randomized studies, highlighting their critical role in medical research. Understanding these studies is essential for anyone involved in clinical research, as they lay the groundwork for valid and reliable outcomes. By defining clear research questions, obtaining ethical approval, and employing robust statistical analysis, researchers can enhance the integrity of their findings.
In the ever-evolving Medtech landscape, randomized studies address key challenges faced by researchers. They not only provide a framework for testing hypotheses but also ensure that the results are applicable to real-world scenarios. This approach fosters trust in clinical research, ultimately benefiting patient care and treatment outcomes.
To successfully execute randomized studies, it is vital to consider several key factors. Researchers must prioritize ethical considerations and engage with relevant stakeholders to navigate the complexities of clinical trials. By doing so, they can ensure that their studies are not only scientifically sound but also ethically responsible.
In conclusion, mastering randomized studies is paramount for advancing clinical research. Collaboration among researchers, institutions, and regulatory bodies is essential to overcome challenges and drive innovation. As you reflect on your own research practices, consider how implementing these strategies can enhance the quality and impact of your work.
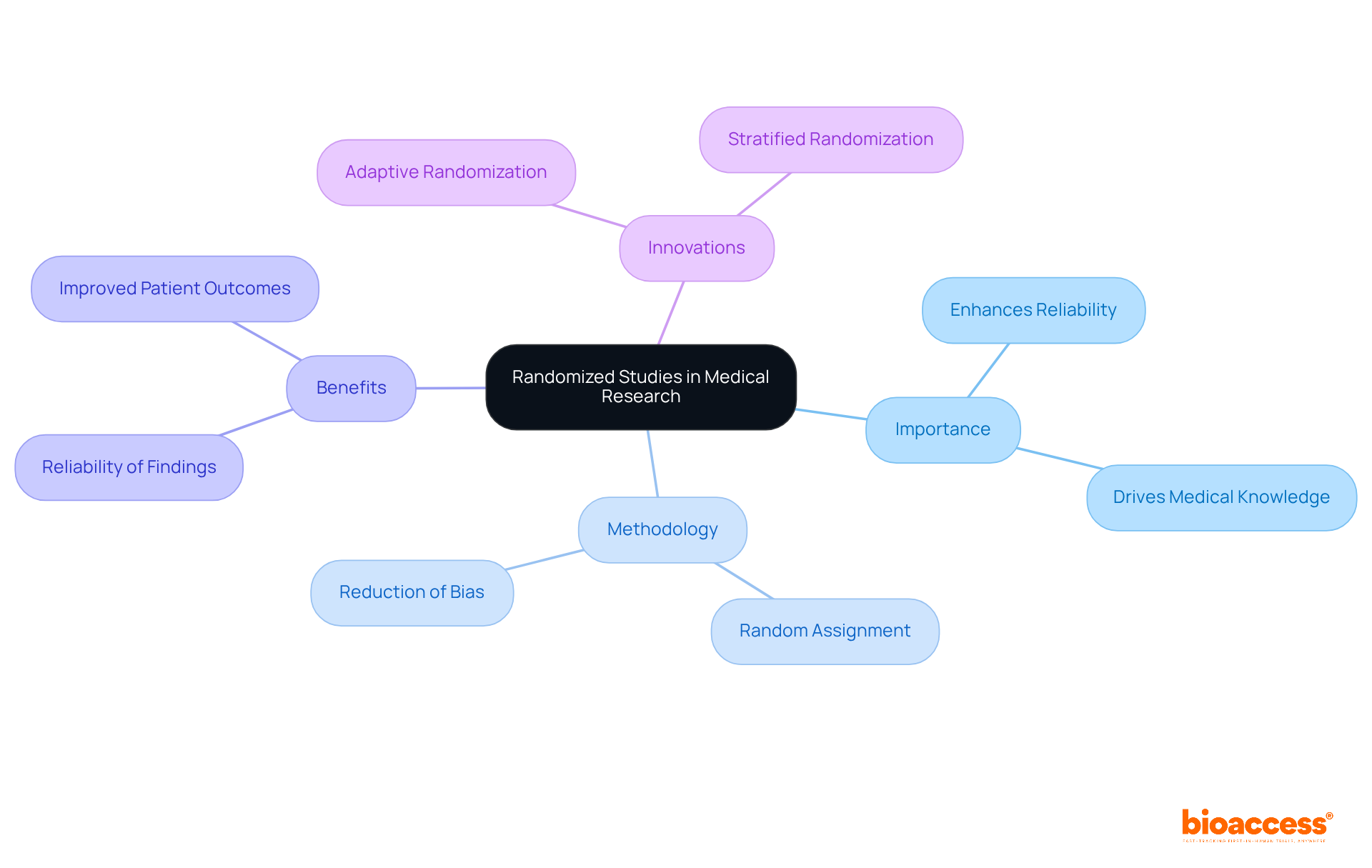
Randomized studies represent the gold standard in medical research, meticulously crafted to eliminate bias and ensure that outcomes can be confidently attributed to specific interventions. This guide explores the intricacies of conducting randomized studies, providing a comprehensive roadmap that equips researchers with essential tools and strategies for success. Given the high stakes in medical advancements, what critical steps and considerations can make or break the integrity of these studies?
Understanding the importance of randomized studies is crucial for anyone involved in clinical research. These studies not only enhance the credibility of findings but also play a pivotal role in advancing medical knowledge. As we delve deeper into the Medtech landscape, it becomes clear that addressing key challenges requires a robust understanding of these methodologies.
In summary, the integrity of randomized studies hinges on careful planning and execution. Collaboration among researchers, clinicians, and stakeholders is vital to navigate the complexities of clinical trials effectively. As we move forward, let’s consider how we can apply these insights to improve our research practices.
A randomized study serves as a cornerstone of medical research, meticulously designed to assess the effectiveness of interventions by randomly assigning participants to distinct groups. This method effectively reduces selection bias, ensuring that groups are comparable and that observed outcomes can be directly attributed to the intervention rather than external factors. The importance of randomization is paramount; it enhances the reliability of findings and drives progress in medical knowledge. For instance, successful randomized studies have shown significant improvements in patient outcomes, with research indicating that a well-executed randomized study method can bolster statistical strength and reduce bias.
Recent innovations in randomization techniques, such as adaptive and stratified randomization, further refine the process, enabling more responsive study designs that cater to diverse patient populations. Experts in the field emphasize that the integrity of randomized study trials is essential for drawing valid conclusions about treatment effects. This integrity ultimately informs healthcare decisions and fosters trust in clinical research. As we navigate the complexities of medical advancements, understanding and implementing robust randomization methods is crucial for advancing patient care.
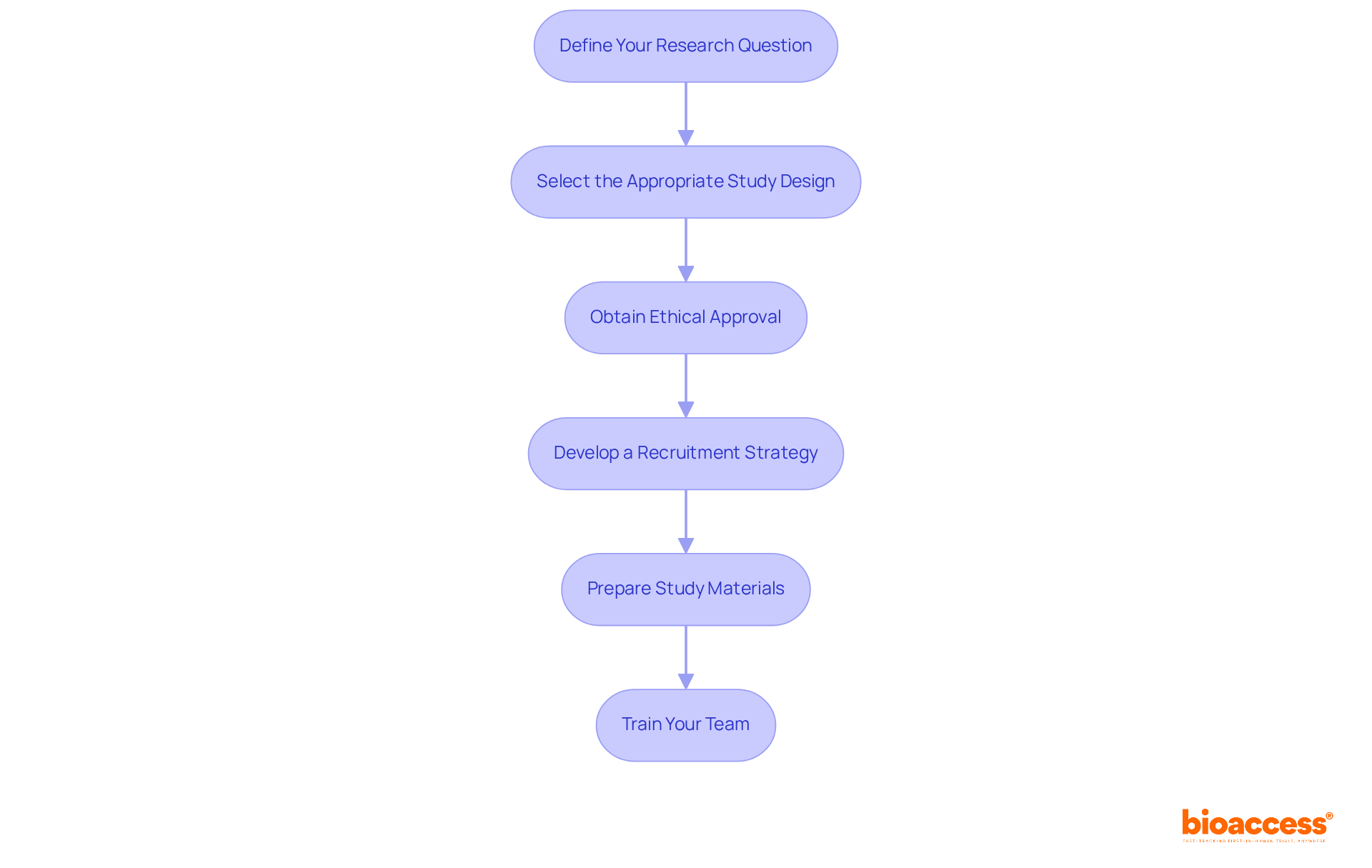
Before launching your randomized study, several key considerations must be addressed:
Define Your Research Question: Clearly articulate the hypothesis you aim to test. This foundational step will guide your research design and methodology, ensuring that your investigation is focused and relevant.
Select the Appropriate Study Design: Choose between parallel-group, crossover, or cluster-randomized designs based on your research question and target population. Each design in a randomized study presents unique advantages and implications for data interpretation.
Obtain Ethical Approval: Submit your research protocol to an Institutional Review Board (IRB) or Ethics Committee. This step is vital, as roughly 20% of research fails to report on individuals involved in a randomized study, emphasizing the significance of ethical adherence in clinical investigations.
Develop a Recruitment Strategy: Identify your target population and create a comprehensive plan for enlisting individuals. Successful recruitment strategies often involve outreach to diverse communities to ensure representation, which is essential for the generalizability of your findings.
Prepare Study Materials: Develop informed consent forms, questionnaires, and any other materials needed for data collection. Clear and concise materials enhance participant understanding and engagement, which is vital for research integrity.
Train Your Team: Ensure that all team members are well-versed in the study protocol and understand their roles and responsibilities. Effective training promotes a united team atmosphere, which is essential for the seamless implementation of the experiment.
Furthermore, utilizing the knowledge of a specialized group, like that provided by bioaccess®, can greatly improve the oversight of your research studies. With more than 20 years of experience in Medtech, bioaccess® offers extensive clinical trial management services, encompassing feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Their emphasis on Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Research guarantees that your project is carried out efficiently and effectively, especially in the Latin American context.
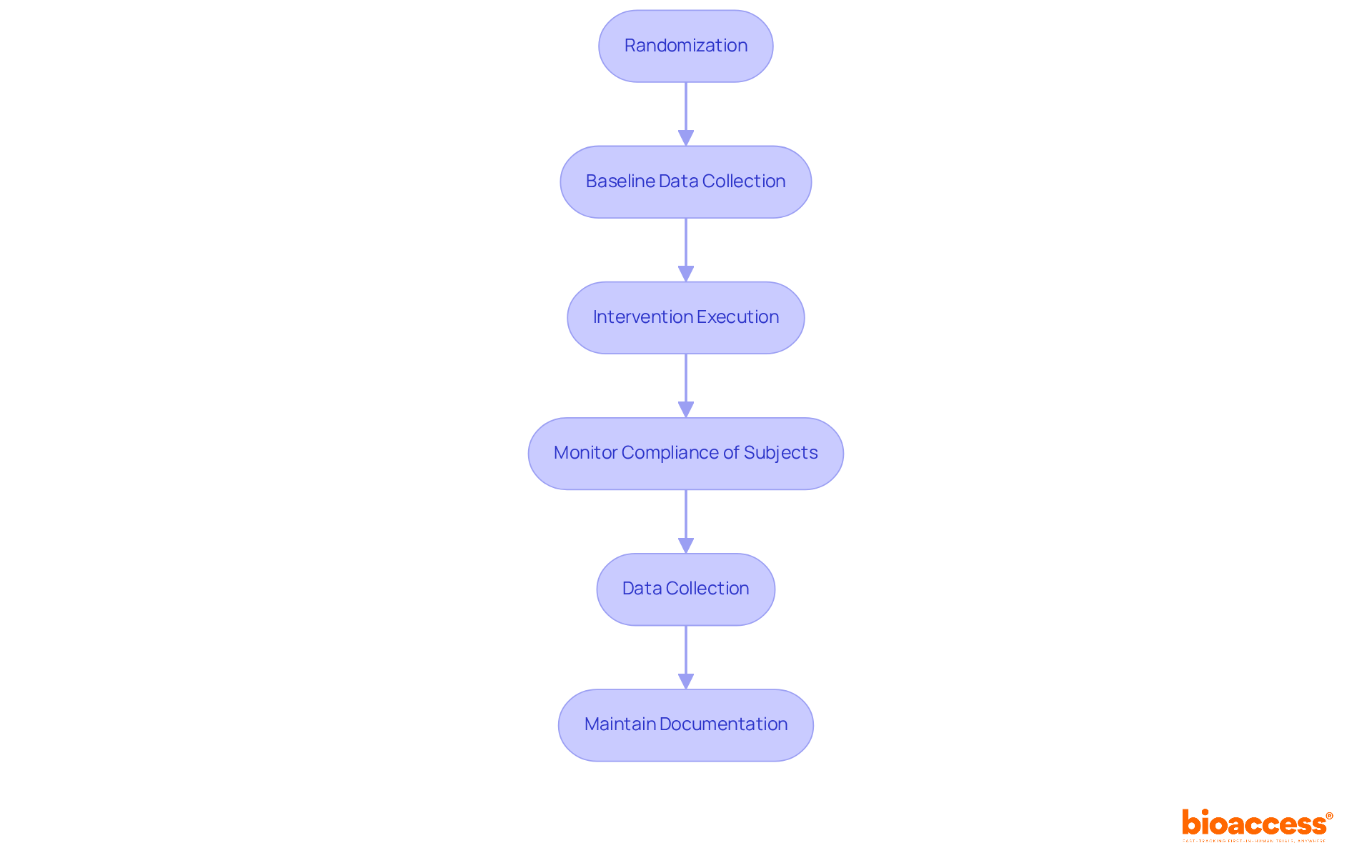
Executing a randomized study is crucial for ensuring the integrity and validity of research findings, especially in the Medtech field. With over 20 years of experience, bioaccess is well-equipped to guide you through this process.
Randomization: A robust randomization process is crucial for assigning individuals to intervention or control groups. Utilizing software tools or random number tables can effectively mitigate selection bias, enhancing the reliability of your results.
Baseline Data Collection: Collecting extensive baseline data from all participants is vital for ensuring comparability between groups. This practice allows for the adjustment of confounding variables during analysis, which is key to obtaining valid outcomes.
Intervention Execution: Administer the intervention according to the research protocol, ensuring uniformity among all participants. Adhering to the protocol is essential for maintaining trial integrity and achieving credible results.
Monitor Compliance of Subjects: Regular communication with subjects is necessary to ensure adherence to the protocol and address any issues that arise. Effective monitoring is critical, as deviations can compromise the validity of the research.
Data Collection: Collect data at predetermined intervals using validated instruments to guarantee accuracy and reliability. This systematic approach captures the necessary information to evaluate the intervention's effectiveness.
Maintain Documentation: Keeping thorough records of all research activities, including participant interactions and data collection, is essential for transparency. Proper documentation facilitates future audits or evaluations of the research.
With bioaccess's tailored support, navigating the complexities of clinical trials in Latin America becomes more manageable.
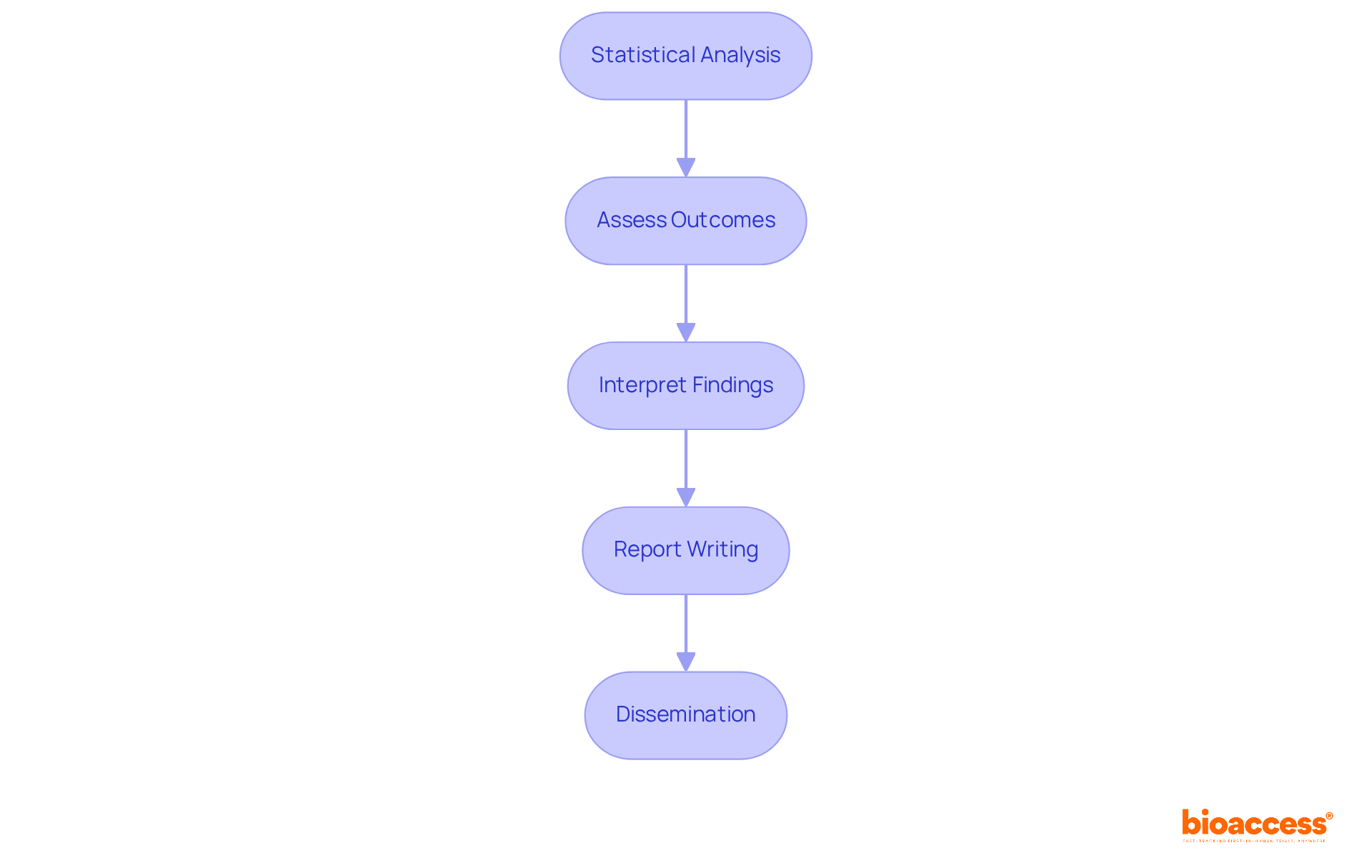
Once your randomized study is complete, analyzing and interpreting the results is essential:
Statistical Analysis: Employ robust statistical techniques tailored to your research's design and sample size. Techniques such as t-tests, ANOVA, and regression analysis are commonly utilized to extract meaningful insights from the data.
Assess Outcomes: Rigorously evaluate both primary and secondary outcomes as outlined in your research protocol. This involves a randomized study that compares results between intervention and control groups to ascertain the treatment's effectiveness.
Interpret Findings: Contextualize your results within the existing body of literature. Discuss how your findings align with or challenge previous studies, thereby enriching the broader understanding of the field.
Report Writing: Craft a comprehensive report that encompasses your methodology, results, and conclusions. Adhere to established guidelines, such as CONSORT, to ensure transparency and reproducibility in your reporting.
Dissemination: Maximize the impact of your research by sharing your findings with the scientific community. Utilize publications, presentations, and conferences to effectively communicate your results and foster further discussion.
Mastering the art of conducting randomized studies is crucial for advancing medical research and enhancing patient outcomes. By grasping the intricacies of randomization and its significance, researchers can design studies that produce reliable and actionable results. The careful planning, execution, and analysis of these studies guarantee that findings are robust and can effectively inform healthcare practices.
This guide has outlined essential steps in preparing for, executing, and analyzing randomized studies. Key considerations, such as:
are vital for ensuring the integrity and validity of the research. Furthermore, leveraging the expertise of organizations like bioaccess® can significantly enhance the management of clinical trials, especially within diverse populations.
Ultimately, the commitment to conducting high-quality randomized studies not only enriches the body of medical knowledge but also plays a pivotal role in shaping future healthcare decisions. Researchers are encouraged to embrace these practices and continuously strive for excellence in their work. The impact of well-conducted studies extends far beyond the confines of the laboratory, influencing the lives of patients and the trajectory of medical advancements.
What is a randomized study?
A randomized study is a type of medical research designed to assess the effectiveness of interventions by randomly assigning participants to different groups, which helps reduce selection bias.
Why is randomization important in medical research?
Randomization is important because it enhances the reliability of findings, ensures that groups are comparable, and allows observed outcomes to be attributed directly to the intervention rather than external factors.
How do randomized studies impact patient outcomes?
Successful randomized studies have demonstrated significant improvements in patient outcomes, indicating that well-executed randomization can bolster statistical strength and reduce bias.
What are some recent innovations in randomization techniques?
Recent innovations include adaptive and stratified randomization, which refine the study process and allow for more responsive designs that cater to diverse patient populations.
What is the role of integrity in randomized study trials?
The integrity of randomized study trials is essential for drawing valid conclusions about treatment effects, which ultimately informs healthcare decisions and fosters trust in clinical research.
How does understanding randomization methods contribute to patient care?
Understanding and implementing robust randomization methods is crucial for advancing medical knowledge and improving patient care as it helps in making informed healthcare decisions.