

This article provides a comprehensive overview of how to master the regulatory pathways for medical technology (medtech) in Mexico. It emphasizes the critical importance of understanding the regulatory framework, particularly the pivotal role of COFEPRIS, as well as the necessary steps for registration and compliance.
By detailing the following aspects, the article serves as an essential guide for manufacturers:
These elements are crucial for effectively navigating the market and ensuring adherence to health standards.
Navigating the complex landscape of medical technology regulations in Mexico is a significant undertaking, particularly as the market continues to expand and evolve. With a projected value of $12.6 billion by 2029, it is essential for manufacturers to grasp the regulatory framework to successfully introduce their products. This article delves into the intricacies of the regulatory pathways for medtech in Mexico, exploring the pivotal role of COFEPRIS and outlining a step-by-step registration process that ensures compliance while accelerating market entry. As new regulations and fast-tracked approval routes emerge, manufacturers must consider:
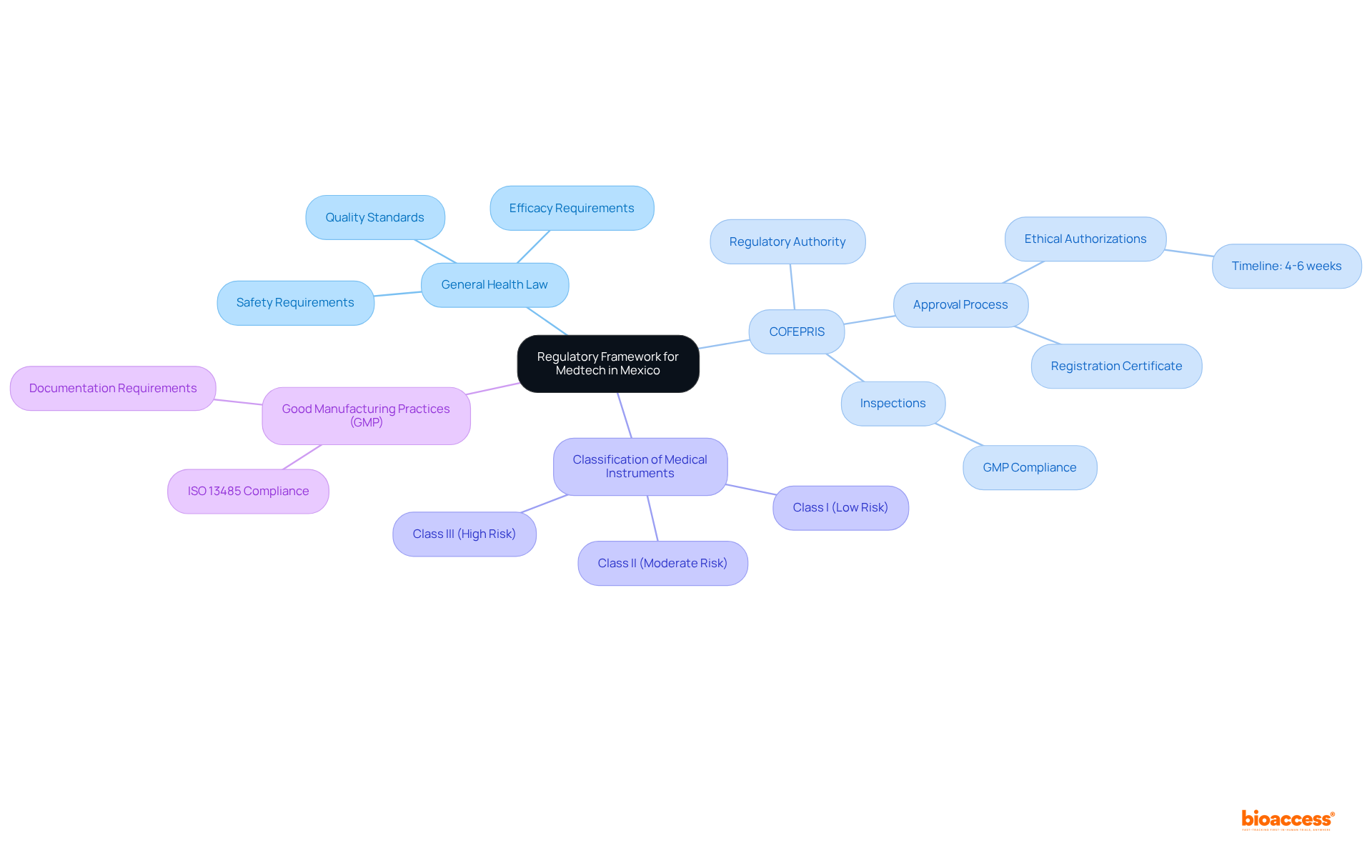
To effectively navigate the regulatory pathways for medtech in Mexico, understanding the key components of the regulatory framework is crucial. The General Health Law (Ley General de Salud) serves as the primary legislation governing health equipment, outlining the essential requirements for safety, efficacy, and quality of health products. Furthermore, the Federal Commission for Protection against Sanitary Risks (COFEPRIS) serves as the regulatory authority responsible for the regulatory pathways for medtech in Mexico.
Familiarity with the categorization of medical instruments, which ranges from low-risk (Class I) to high-risk (Class III), is vital, as this classification dictates the filing process and requirements. In Mexico, the approval process for medical equipment typically spans 4-6 weeks for ethical authorizations, underscoring the efficiency of a procedure in a market valued at 9.4 billion USD.
Manufacturers must also adhere to Good Manufacturing Practices (GMP) and furnish documentation, including ISO 13485 quality management system certificates. Understanding the regulatory pathways for medtech in Mexico is essential for preparing for the subsequent steps in the registration process.
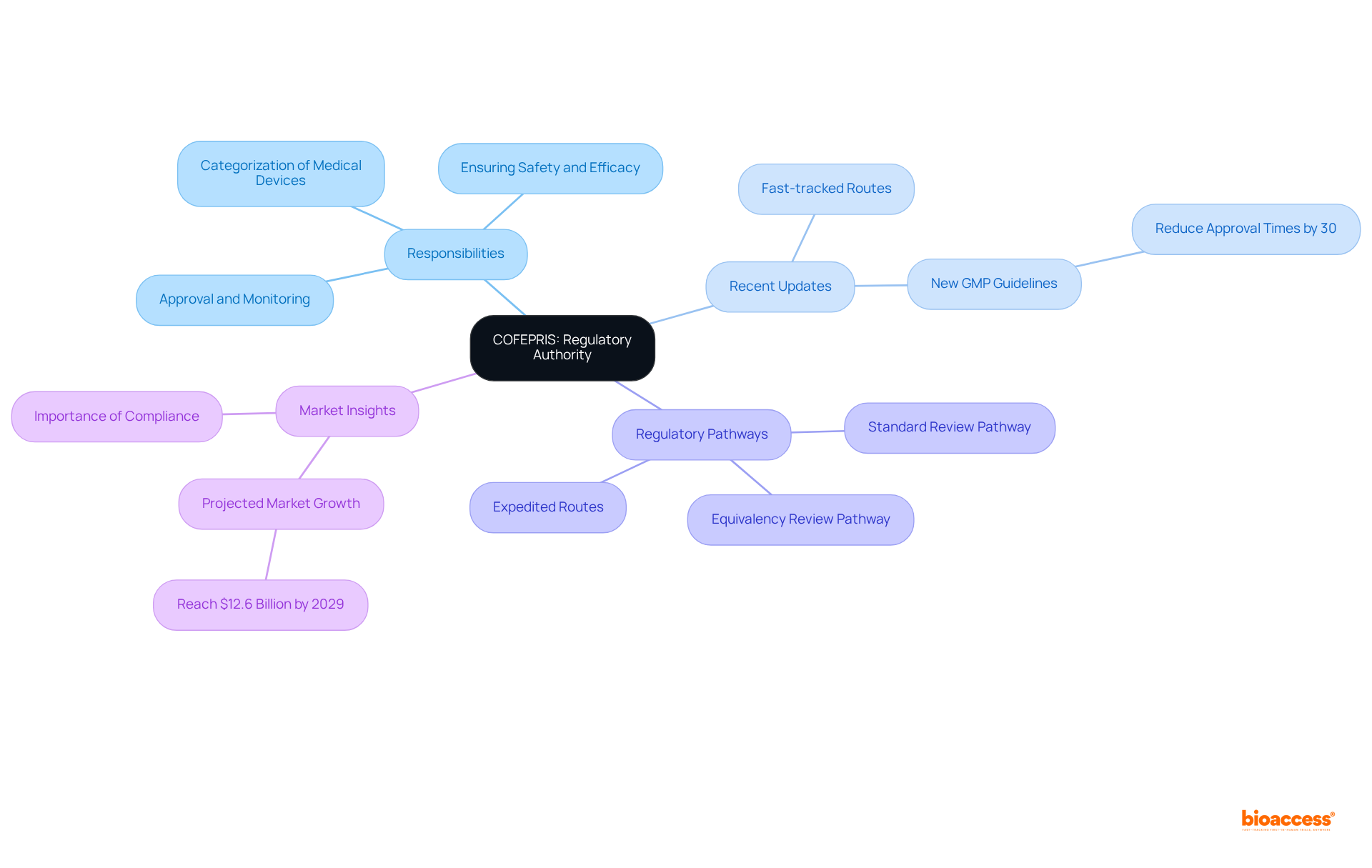
The Federal Commission for Protection against Sanitary Risks serves as the regulatory authority overseeing the regulatory pathways for medtech in Mexico, including the approval and monitoring of healthcare instruments. Operating under the Ministry of Health, it bears the critical responsibility of ensuring that all health products adhere to established safety and efficacy standards. Understanding the agency's structure, including its various departments that manage different facets of the regulatory pathways for medtech in Mexico, is essential for effective interaction with COFEPRIS.
Recent updates, particularly the introduction of fast-tracked routes for products already authorized in other regions, can significantly influence your approval strategy. Familiarizing yourself with COFEPRIS's guidelines is imperative, as they provide insight into the regulatory pathways for medtech in Mexico, detailing the necessary documentation and processes for successful registration. Notably, the new Good Manufacturing Practices (GMP) guidelines, which are anticipated to reduce approval times by as much as 30%, represent a substantial update that manufacturers should carefully consider.
Furthermore, comprehending the categorization of healthcare instruments based on intended use and risk is vital for effectively navigating the regulatory pathways for medtech in Mexico. The healthcare equipment market in Mexico is projected to reach $12.6 billion by 2029, underscoring the importance of timely adherence to regulations and strategic market entry. Appointing a qualified Sanitary Responsible is equally critical for managing regulatory affairs in Mexico, ensuring compliance with all necessary regulations.
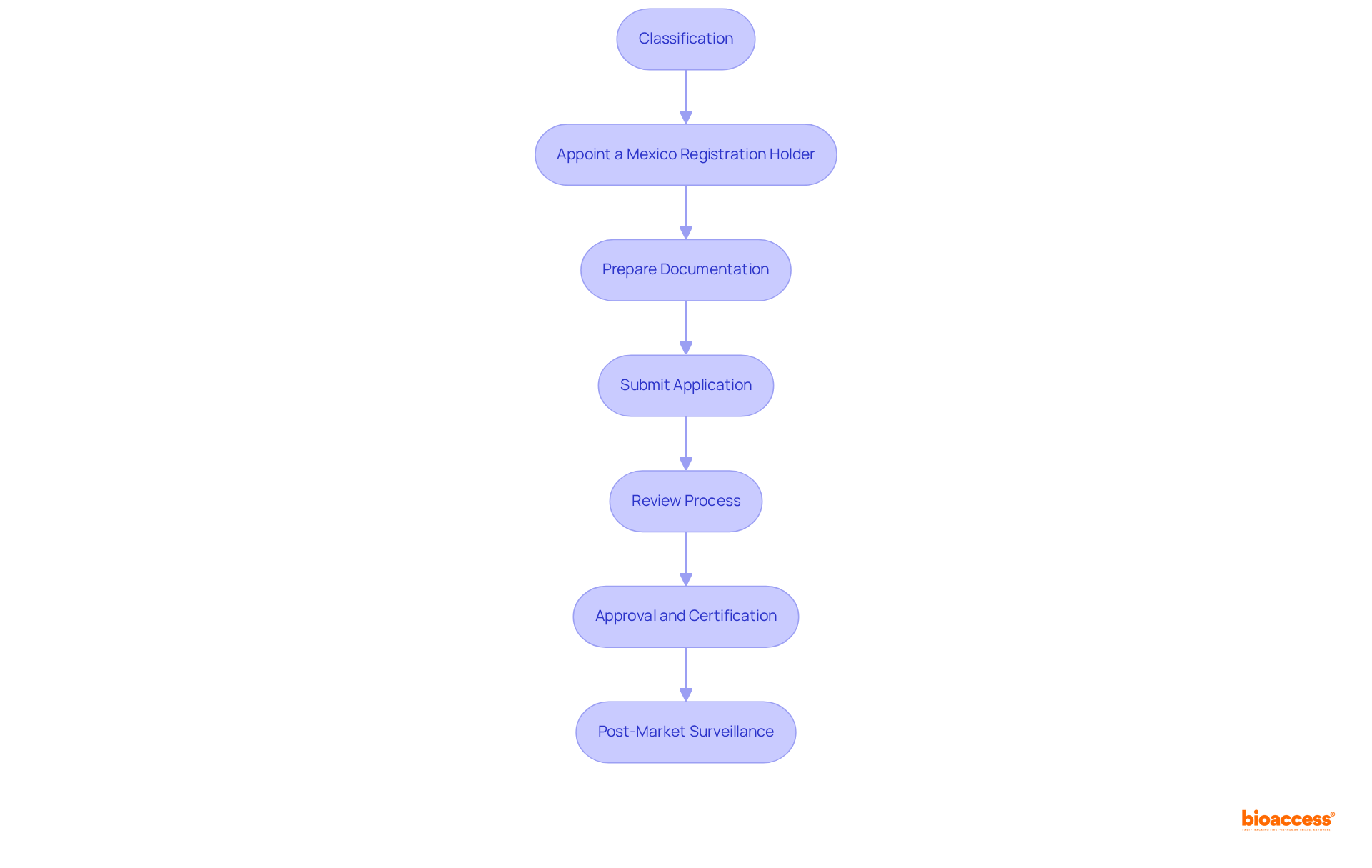
The registration process for medical devices in Mexico involves several critical steps that must be followed meticulously:
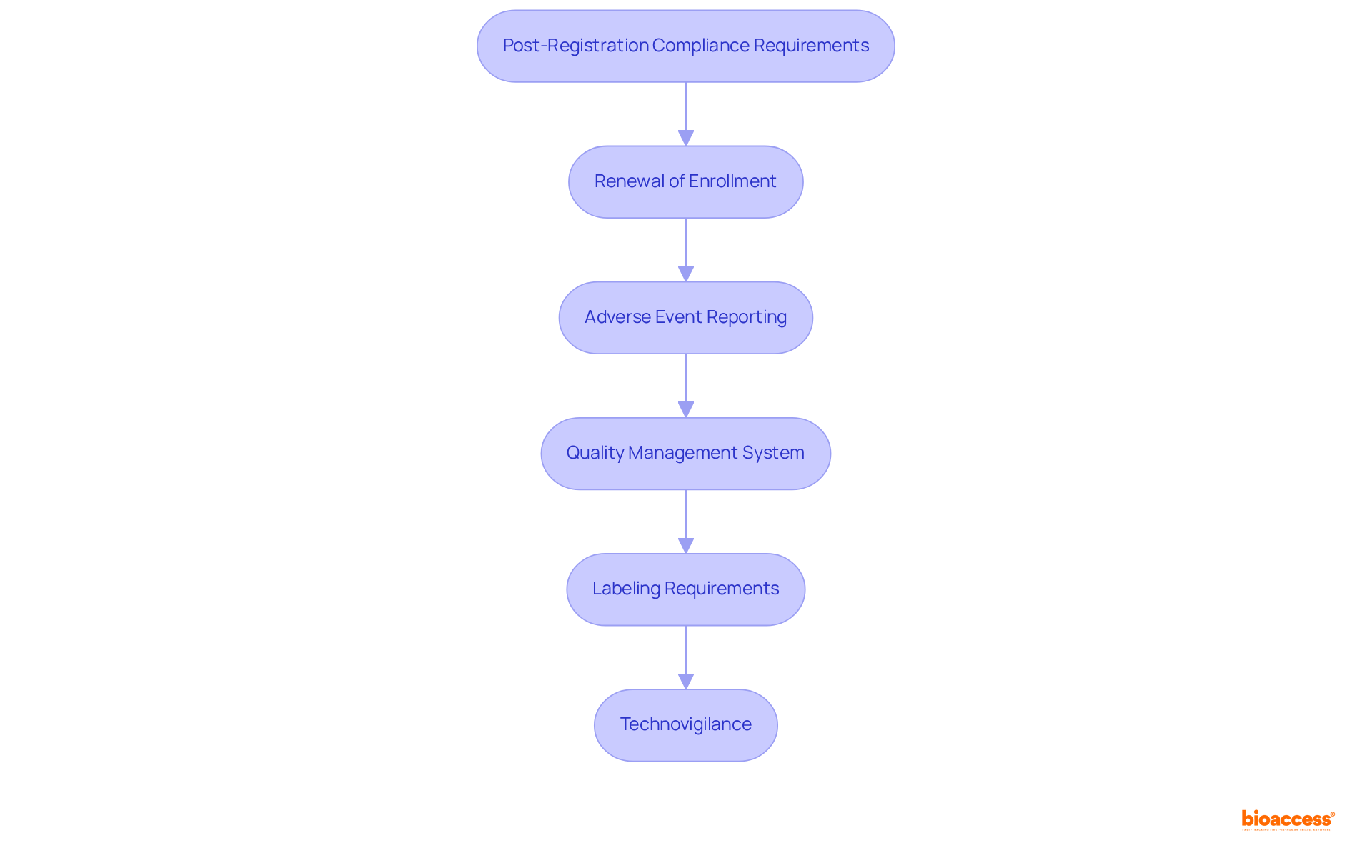
Once your medical equipment is registered with COFEPRIS, adhering to post-registration compliance requirements is essential for maintaining market access. Key obligations include:
By fulfilling these post-registration obligations, you can ensure the continued success and compliance of your medical device within the regulatory pathways for medtech in Mexico.
Navigating the regulatory pathways for medtech in Mexico necessitates a comprehensive grasp of the framework established by the General Health Law and the essential role of COFEPRIS. Mastery of these regulations is crucial for ensuring the safety, efficacy, and quality of medical devices, thereby facilitating successful market entry in this rapidly growing sector.
Key insights from the article underscore the significance of:
The step-by-step registration process, along with adherence to post-registration compliance obligations, highlights the imperative for manufacturers to remain informed about evolving guidelines and timelines, especially as the market is projected to expand significantly by 2029.
In summary, the medtech landscape in Mexico offers both opportunities and challenges. By comprehending the regulatory framework and proactively engaging with COFEPRIS, manufacturers can streamline their approval processes and ensure compliance. This strategic approach not only enhances the likelihood of successful market entry but also contributes to the overall safety and quality of healthcare products available to the Mexican population.
What is the primary legislation governing health equipment in Mexico?
The primary legislation governing health equipment in Mexico is the General Health Law (Ley General de Salud).
Who is the regulatory authority responsible for medtech in Mexico?
The Federal Commission for Protection against Sanitary Risks (COFEPRIS) is the regulatory authority responsible for the regulatory pathways for medtech in Mexico.
How are medical instruments categorized in Mexico?
Medical instruments in Mexico are categorized into three classes: low-risk (Class I), moderate-risk (Class II), and high-risk (Class III).
What is the typical approval process duration for medical equipment in Mexico?
The approval process for medical equipment in Mexico typically spans 4-6 weeks for ethical authorizations.
What are Good Manufacturing Practices (GMP) in the context of medtech?
Good Manufacturing Practices (GMP) are standards that manufacturers must adhere to ensure the safety, efficacy, and quality of health products.
What documentation must manufacturers provide during the registration process?
Manufacturers must provide documentation, including ISO 13485 quality management system certificates, during the registration process.
Why is understanding the regulatory pathways important for medtech manufacturers in Mexico?
Understanding the regulatory pathways is essential for preparing for the subsequent steps in the registration process and ensuring compliance with health regulations.