

The article elucidates the significance of mastering the timeline of drug development, which encompasses essential stages from discovery to post-marketing surveillance, typically spanning 10 to 15 years. It captures interest by detailing the complexities and challenges encountered at each stage, such as the high failure rates in clinical trials and the implications of regulatory requirements. Furthermore, it generates desire by suggesting strategies to expedite the process, including adaptive trial designs and early engagement with regulators. This comprehensive overview reinforces the conviction that understanding these elements is crucial for success in clinical research. Ultimately, it prompts action by encouraging stakeholders to consider how they can navigate these challenges effectively.
The intricate journey of drug development represents a monumental task, transforming innovative scientific ideas into life-saving medications. Spanning an estimated 10 to 15 years, this complex timeline encompasses multiple stages, each fraught with challenges and opportunities for advancement. As the pharmaceutical industry confronts increasing pressures to expedite the development process, a crucial question emerges: what strategies can be employed to navigate these hurdles and enhance efficiency in bringing new therapies to market?
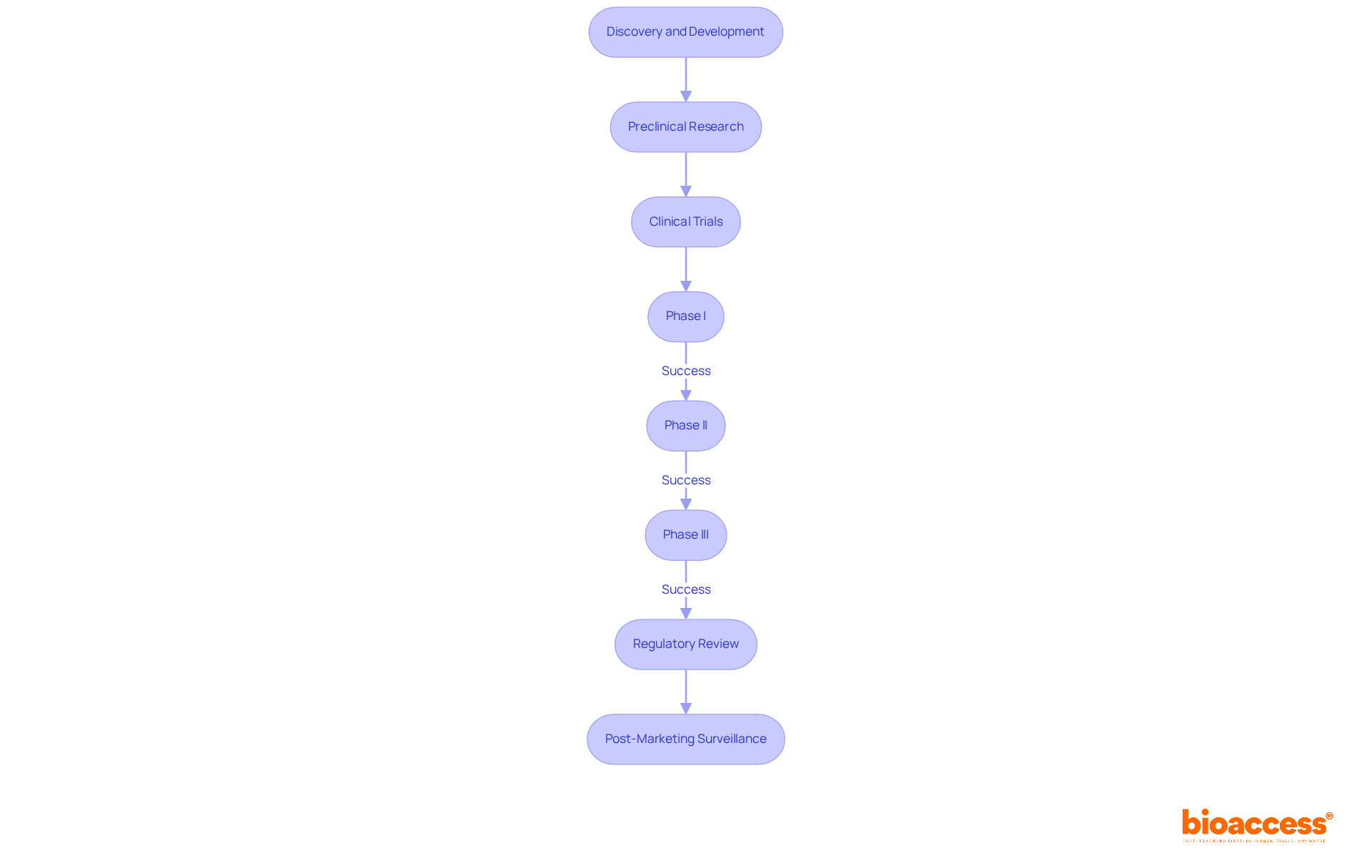
The journey of pharmaceutical development follows a complex timeline of drug development that transforms scientific discoveries into marketable medications. This process typically encompasses several key stages:
Discovery and Development: This initial phase focuses on identifying potential medication candidates through extensive laboratory research and preclinical testing, which can take 4 to 7 years. Approximately 90% of medications entering clinical trials fail, underscoring the challenges inherent in this early stage.
Preclinical Research: Before human testing, drugs undergo rigorous laboratory and animal studies to assess safety and biological activity. This phase is crucial for determining whether a compound is viable for further development.
Clinical Trials: This phase is divided into three main stages:
Regulatory Review: Following successful clinical trials, a New Drug Application (NDA) or Biologics License Application (BLA) is submitted to regulatory bodies like the FDA. The review process typically takes 6 to 10 months, with expedited pathways significantly shortening this timeline for eligible therapies.
Post-Marketing Surveillance: Once a medication is available, ongoing monitoring is essential to ensure long-term safety and effectiveness. Phase IV studies may be required by regulatory bodies to collect further safety information and evaluate interactions in real-world environments.
The timeline of drug development from medicine discovery to market approval typically spans approximately 10 to 15 years and is influenced by various factors including the complexity of the medication, patient recruitment challenges, and regulatory requirements. Firms that strategically incorporate accelerated routes into their development strategies, such as those provided by bioaccess®, are better equipped to navigate this extensive journey and enhance their market preparedness.
The drug development process represents a structured journey, segmented into several critical phases:
Preclinical Stage: This initial period encompasses laboratory and animal research aimed at evaluating the medication's safety profile and biological activity, laying the foundation for subsequent human studies.
Stage I Studies: Focusing primarily on safety, these studies assess the medication's pharmacokinetics and pharmacodynamics in a small cohort of healthy participants. Stage I studies, which typically last several months, are vital for identifying potential adverse effects in the timeline of drug development.
Stage II Evaluations: Conducted with a larger group of individuals affected by the condition the medication targets, Stage II evaluations examine the drug's effectiveness and side effects. The duration of this phase within the timeline of drug development may extend from several months to a couple of years, depending on the substance and condition under investigation.
Stage III Studies: These extensive studies involve hundreds to thousands of participants and aim to confirm effectiveness, monitor side effects, and compare the new medication with commonly used therapies. The complexity and scale of Phase III studies can extend the timeline of drug development, resulting in durations that span several years.
Regulatory Submission: Following the successful completion of trials, the collected data is compiled into a New Drug Application (NDA), which is an important step in the timeline of drug development for review by regulatory authorities. This phase is crucial for obtaining approval to market the medication.
Post-Marketing Phase: Once a medication receives approval, ongoing monitoring becomes essential. This phase involves tracking the drug's performance in the general population, reporting adverse events, and conducting further studies to ensure continued safety and efficacy.
Recent insights reveal that 65% of clinical study sites acknowledge a lack of real-time data access as a significant barrier to execution, while 78% experience delays stemming from poor communication. These findings underscore the importance of efficient operations and swift adaptation to sponsor requirements for the successful execution of studies. Furthermore, the integration of artificial intelligence and machine learning has the potential to reduce study timelines by as much as 30%, thereby enhancing the overall effectiveness of drug development.
bioaccess® streamlines this process through its comprehensive clinical study management services, which encompass feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. This integrated approach not only accelerates regulatory approval but also facilitates patient enrollment at a rate 50% faster, resulting in substantial cost savings of $25K per patient with FDA-ready data. Additionally, 72% of sponsors believe that enhanced training would significantly improve site performance, highlighting the necessity for improved communication and operational strategies in clinical studies.

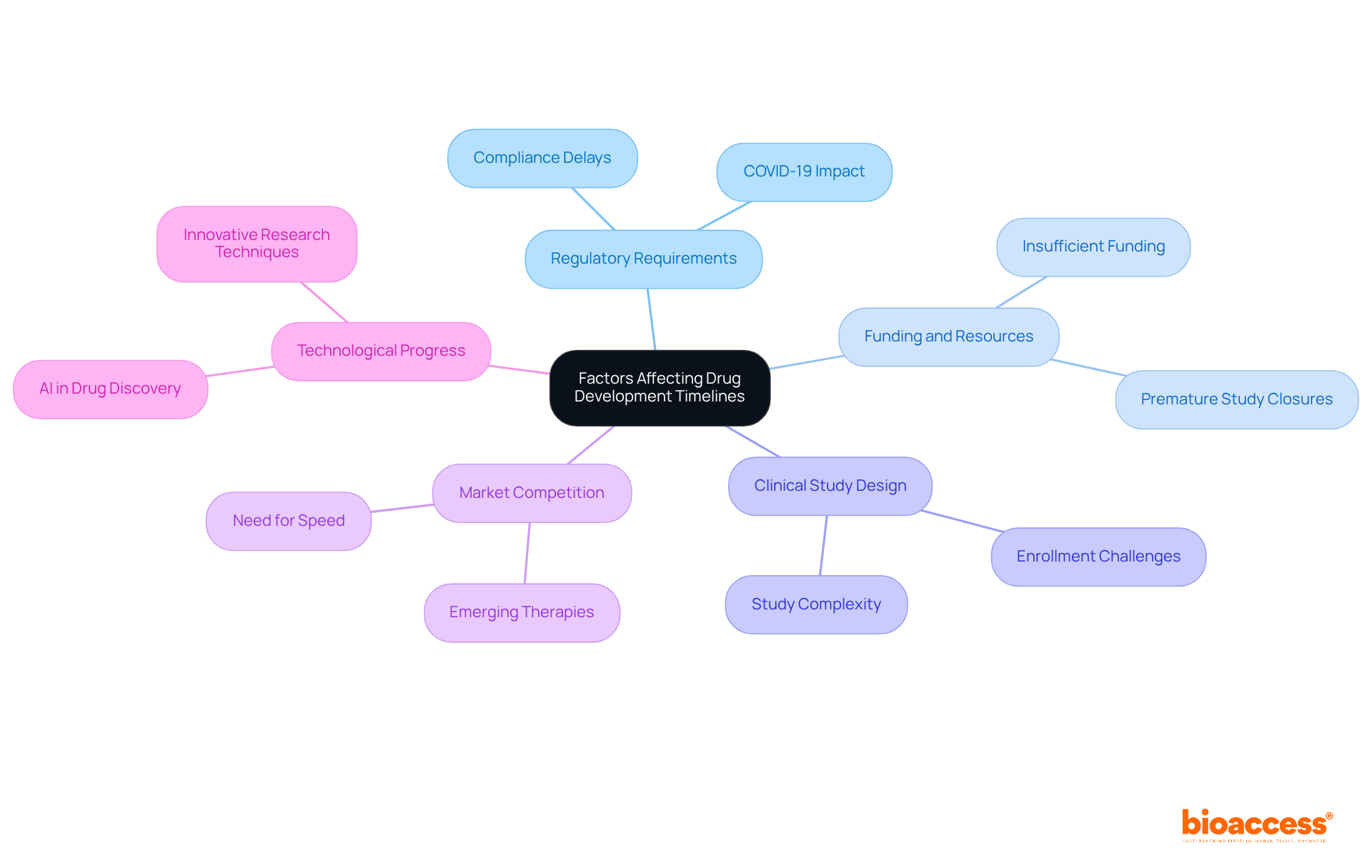
Several factors significantly influence the timelines of drug development, including:
Regulatory Requirements: Compliance with local and international regulations can introduce delays, particularly when additional data or studies are mandated. The COVID-19 pandemic underscored the necessity for larger, more coordinated clinical studies, as many were too small and lacked statistical power, resulting in inefficiencies in drug approval processes.
Funding and Resources: The availability of financial resources is crucial, as insufficient funding can impede research activities. Approximately 19% of clinical studies conclude prematurely due to insufficient participants, leading to an estimated $800 billion loss in value. This emphasizes the essential requirement for sufficient funding to support robust clinical study designs and patient recruitment strategies throughout the timeline of drug development.
Clinical Study Design: The intricacy of study designs, including the number of locations and patient recruitment strategies, can significantly influence timelines. On average, the timeline of drug development shows that it takes about 19 months to enroll patients in trials, with around 85% not starting on time due to enrollment issues. This highlights the importance of optimized procedures to enhance efficiency within the timeline of drug development.
Market Competition: The presence of rival medications can accelerate the need for quicker development to capture market share. As the pharmaceutical landscape evolves, companies must adapt swiftly within the timeline of drug development to maintain a competitive edge, particularly in the face of emerging therapies and technologies.
Technological Progress: Innovations in research techniques and technologies can simplify workflows, potentially shortening medication development timelines. For instance, the incorporation of artificial intelligence in medication discovery is anticipated to reduce two years from the usual ten-year timeline of drug development, showcasing the revolutionary capabilities of technology in the field.
To expedite the drug development process, consider implementing the following strategies:
By implementing these strategies, organizations can navigate the complexities of the timeline of drug development more effectively, ultimately leading to quicker access to innovative therapies for patients.

The intricate timeline of drug development represents a transformative journey, turning scientific innovation into life-saving medications, typically spanning 10 to 15 years. This process encompasses several critical stages:
Each phase presents unique challenges and opportunities, underscoring the necessity of strategic planning and execution to navigate the complex landscape of pharmaceutical development.
Key insights throughout the article illuminate the rigorous nature of each stage, from the high failure rates during initial discovery to the stringent requirements of regulatory review. Factors such as funding, clinical study design, and technological advancements significantly influence timelines. Moreover, strategies like adaptive trial designs and early regulator engagement can enhance efficiency. Companies that leverage innovative solutions, such as those offered by bioaccess®, position themselves advantageously to overcome obstacles and expedite the journey from laboratory to market.
Ultimately, grasping the timeline of drug development is crucial for stakeholders eager to bring new therapies to patients more efficiently. By embracing best practices and fostering collaboration across disciplines, the pharmaceutical industry can continue to evolve, ensuring that groundbreaking treatments reach those in need without unnecessary delays. The commitment to innovation and improvement in drug development processes is essential for advancing healthcare and enhancing patient outcomes.
What are the main stages of drug development?
The main stages of drug development include Discovery and Development, Preclinical Research, Clinical Trials (which are divided into Phase I, Phase II, and Phase III), Regulatory Review, and Post-Marketing Surveillance.
How long does the drug development process typically take?
The drug development process typically spans approximately 10 to 15 years.
What happens during the Discovery and Development phase?
This phase focuses on identifying potential medication candidates through extensive laboratory research and preclinical testing, which can take 4 to 7 years.
What is involved in Preclinical Research?
Preclinical Research involves rigorous laboratory and animal studies to assess the safety and biological activity of the drug before it undergoes human testing.
What are the three stages of Clinical Trials?
The three stages of Clinical Trials are: Phase I: Assessing safety and tolerability on 20 to 80 healthy volunteers, lasting up to one year. Phase II: Evaluating efficacy and safety in 100 to 300 patients diagnosed with the target disease, typically lasting up to two years. Phase III: Conducting extensive studies with a minimum of 1,000 patients over one to four years to prove safety and effectiveness.
What is the success rate of drugs advancing through the clinical trial phases?
Approximately 60-70% of compounds advance from Phase I to Phase II, 30-35% from Phase II to Phase III, and only 25-30% of drugs entering Phase III succeed.
What is the purpose of the Regulatory Review stage?
The Regulatory Review stage involves submitting a New Drug Application (NDA) or Biologics License Application (BLA) to regulatory bodies like the FDA for approval, which typically takes 6 to 10 months.
What is Post-Marketing Surveillance?
Post-Marketing Surveillance involves ongoing monitoring of a medication after it becomes available to ensure long-term safety and effectiveness, often requiring Phase IV studies to collect further safety information.
How do companies like bioaccess® assist in the drug development process?
Companies like bioaccess® provide specialized services such as regulatory approval, clinical research site initiation, and patient enrollment, which can expedite clinical studies and improve recruitment duration and retention rates.