


Mastering TMF (Trial Master File) clinical trial management is essential for ensuring compliance and data integrity throughout clinical studies. This article underscores the critical role of effective TMF management, which necessitates robust documentation practices and the strategic use of technology to enhance efficiency. By adhering to best practices, organizations can collectively boost the credibility and success rates of clinical trials, fostering a more reliable research environment.
The Trial Master File (TMF) stands as the cornerstone of clinical trial management, encompassing the essential documents that uphold compliance and data integrity. As the clinical research landscape continues to evolve, the demand for robust TMF management strategies intensifies—strategies that not only streamline processes but also protect against regulatory pitfalls. With the increasing emphasis on transparency and accountability, organizations must consider:
The TMF clinical trial is a crucial repository of essential documents that underpins the management and oversight of clinical studies. It encompasses all documentation necessary to demonstrate compliance with regulatory standards set forth by organizations such as the EMA and FDA, while also preserving the integrity of research data.
Functioning as a legal record of the conduct of proceedings, the TMF ensures transparency and accountability—elements that are vital for fostering trust among stakeholders. A meticulously maintained TMF is indispensable for successful audits and inspections, significantly influencing the credibility of the study and the safety of participants.
For instance, timely uploads of study documents to the TMF can prevent last-minute scrambles before inspections, thereby reducing stress and enhancing compliance. Conversely, the lack of a comprehensive TMF can expose studies to the risks of non-compliance, leading to delays, increased costs, and potential legal ramifications.
As regulatory bodies enforce more stringent standards, the TMF clinical trial's role in safeguarding data integrity and ensuring adherence to regulations has never been more critical, directly impacting the success rates of clinical research.

Effective management of the Trial Master File (TMF) is essential for the success of clinical trials and involves several key components:
Record Control: A robust record management system is vital for preserving version integrity. This includes ensuring that all files are current, accessible, and properly archived. Statistics indicate that organizations with efficient record management systems experience a 30% decrease in regulatory issues during audits.
Vital Papers: Maintaining a comprehensive list of vital papers, including the study protocol, informed consent forms, and regulatory approvals, is crucial. Creating a specific list for the study at the trial's outset streamlines the documentation process and guarantees adherence to regulatory requirements.
Audit Trails: The implementation of audit trails is necessary for tracking changes and updates to documents. This practice provides a transparent history of modifications, which is critical during inspections. Regular audits can reveal that up to 40% of discrepancies in the TMF clinical trial arise from inadequate documentation practices.
Training and Regulations: All team members involved in TMF management must undergo thorough training on best practices and regulatory adherence. This training fosters a culture of accountability and ensures that everyone comprehends their role in maintaining TMF quality. Regular training sessions on TMF clinical trial quality and responsibilities are essential for compliance.
Regular Reviews: Conducting periodic evaluations of the TMF is essential for identifying gaps and ensuring that all necessary records are included and organized. A proactive review timetable can significantly enhance the TMF's reliability, with studies indicating that regular evaluations can improve record completeness by over 25%. Furthermore, applying a TMF completeness checklist derived from the TMF Reference Model can assist in verifying that all anticipated materials are included for the TMF clinical trial.
By concentrating on these components, organizations can elevate their TMF management processes, ensuring compliance and facilitating smoother audits and inspections.

Leveraging technology is essential for streamlining processes in a TMF clinical trial within clinical research. Consider the following strategies:

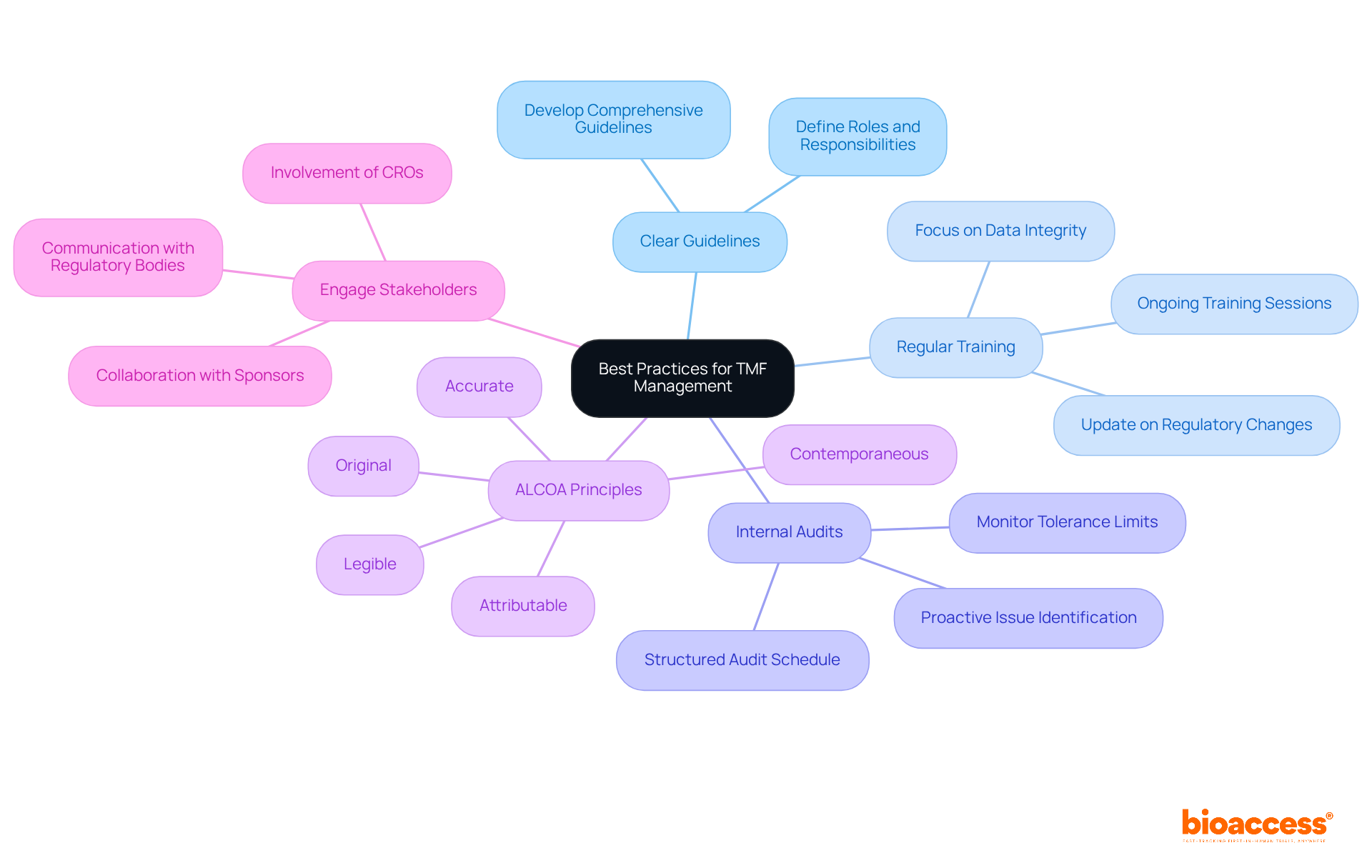
To ensure quality and compliance in TMF management, it is imperative to consider the following best practices:
Establish Clear Guidelines: Develop and communicate comprehensive guidelines for TMF management, detailing roles and responsibilities for all team members. This clarity streamlines processes and enhances accountability.
Regular Training: Conduct ongoing training sessions to keep staff informed about TMF processes, regulatory updates, and best practices. Ongoing education is essential, as nearly 80% of data integrity-related warning letters from the FDA since 2008 were issued during 2014-2018, underscoring the necessity for vigilance in adherence.
Conduct Internal Audits: Implement a structured schedule for internal audits of the TMF. These audits are essential for proactively identifying and correcting regulatory issues, ensuring that organizations are prepared for external inspections. Effective monitoring of tolerance limits is crucial for maintaining TMF integrity.
Focus on ALCOA Principles: Ensure that all documents adhere to the ALCOA principles—Attributable, Legible, Contemporaneous, Original, and Accurate. These principles are vital for maintaining data integrity and compliance with Good Clinical Practice (GCP) standards, as they help prevent data misinterpretation and manipulation.
Engage Stakeholders: Foster collaboration among all parties involved in the study, including sponsors, CROs, and regulatory bodies. This engagement is key to ensuring comprehensive TMF clinical trial management and oversight, promoting transparency and accountability across all parties involved in the process.
The significance of mastering Trial Master File (TMF) management in clinical trials cannot be overstated. A well-organized TMF serves as the backbone of compliance and data integrity, ensuring that all essential documents are readily available and transparent. This meticulous approach enhances the credibility of clinical studies and fosters trust among stakeholders, ultimately contributing to the overall success of research endeavors.
Key strategies for effective TMF management include:
By focusing on these components, organizations can significantly reduce regulatory issues and enhance their readiness for inspections. Additionally, adopting best practices such as regular training, internal audits, and adherence to ALCOA principles further reinforces the quality and compliance of TMF management.
In light of the increasing regulatory demands and the complexity of clinical trials, the call to action is clear: organizations must prioritize TMF management to safeguard data integrity and ensure compliance. Embracing modern technology and best practices will not only streamline TMF processes but also pave the way for successful clinical trials in the ever-evolving landscape of clinical research.
What is the Trial Master File (TMF) in clinical trials?
The TMF is a crucial repository of essential documents that supports the management and oversight of clinical studies, demonstrating compliance with regulatory standards.
Why is the TMF important in clinical trials?
The TMF is important because it serves as a legal record of the conduct of clinical trials, ensuring transparency and accountability, which are vital for building trust among stakeholders.
How does a well-maintained TMF affect audits and inspections?
A meticulously maintained TMF is essential for successful audits and inspections, influencing the credibility of the study and the safety of participants.
What are the consequences of not having a comprehensive TMF?
The absence of a comprehensive TMF can lead to non-compliance risks, resulting in delays, increased costs, and potential legal issues.
How does timely document management in the TMF benefit clinical trials?
Timely uploads of study documents to the TMF can prevent last-minute scrambles before inspections, reducing stress and enhancing compliance.
What impact do regulatory standards have on the TMF's role?
As regulatory bodies enforce stricter standards, the TMF's role in safeguarding data integrity and ensuring adherence to regulations has become increasingly critical, directly affecting the success rates of clinical research.