


The article emphasizes effective strategies for managing treatment and control in clinical trials, highlighting the critical role of:
It details best practices such as:
By addressing these key areas, the article underscores the importance of collaboration and proactive measures in navigating the complexities of clinical research.
Navigating the complex landscape of clinical trials necessitates a profound understanding of regulatory compliance, effective participant recruitment, and the strategic management of multiple research projects. Mastering these elements enables organizations to enhance the credibility of their studies and improve overall outcomes. However, as regulations evolve and the demand for diverse patient populations intensifies, how can research teams ensure they are equipped to confront these challenges head-on? This article explores best practices and innovative strategies that empower clinical trial professionals to optimize treatment control and achieve successful results in their research endeavors.
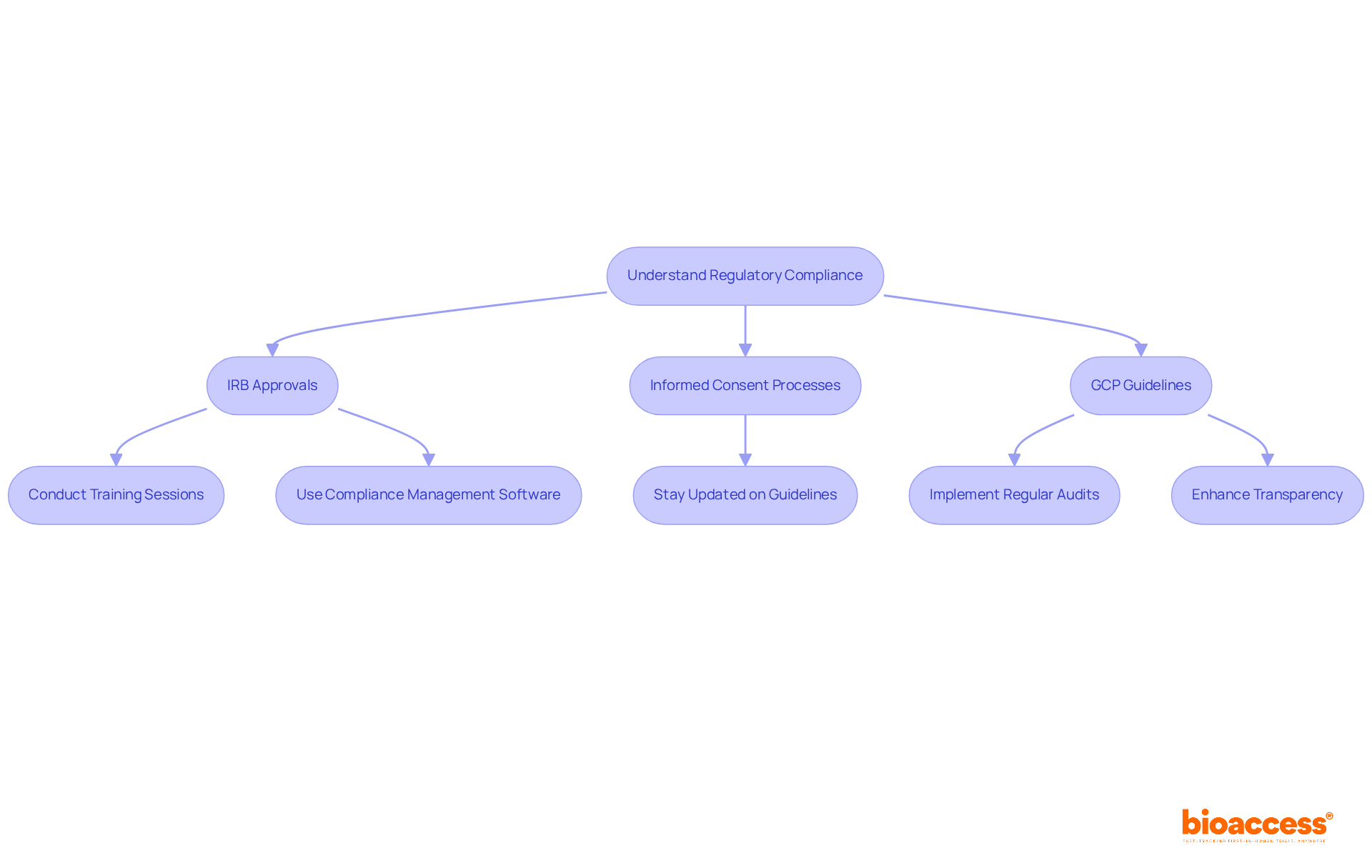
To efficiently manage regulatory adherence in research studies, it is crucial to remain informed about the latest guidelines from regulatory bodies such as the FDA and EMA. This includes a thorough understanding of the requirements for:
Regular training sessions for the research team are essential to ensure that all members are cognizant of their compliance responsibilities. Furthermore, the use of compliance management software can streamline the tracking of regulatory documents and deadlines, significantly mitigating the risk of oversight.
Recent updates from the EMA underscore the importance of transparency and prompt reporting in research studies. The Clinical Trials Regulation (CTR) mandates that all research studies must be registered in the Clinical Trials Information System (CTIS) prior to initiation. This regulation aims to enhance public access to examination information and bolster accountability among sponsors. Additionally, adherence to the FDAAA 801 Final Rule, which necessitates the registration and reporting of clinical trial results, is paramount. Organizations that implement a robust compliance strategy—including regular audits and updates—can avert costly delays and enhance the credibility of their research outcomes. Indeed, studies indicate that compliance failures can lead to substantial penalties and loss of funding, highlighting the critical need for maintaining rigorous compliance practices.
To optimize participant recruitment, crafting a robust strategy is essential. This strategy must encompass outreach to healthcare providers, community organizations, and patient advocacy groups. By leveraging digital marketing techniques—such as targeted social media campaigns—research teams can significantly enhance their ability to connect with potential participants. Additionally, streamlining the enrollment procedure and clearly conveying the advantages of the study are crucial for increasing interest and participation.
A recent partnership between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, aims to establish Barranquilla as a premier location for research studies in Latin America. This collaboration exemplifies how strategic alliances can bolster recruitment efforts and streamline processes. For instance, a research study that partnered with local health clinics achieved an impressive 30% rise in enrollment rates. This strategy not only expanded the participant pool but also fostered trust within the community, resulting in improved retention rates throughout the study.
Moreover, Medtech and Biopharma startups often face challenges in securing healthcare provider participation, which can hinder recruitment efforts. As the research environment evolves, incorporating digital marketing into recruitment strategies becomes increasingly vital for success. It is also important to note that 30% of patients drop out of a trial before it launches, highlighting the challenges of maintaining participant engagement. As Cara Brant, CEO of Clinical Trial Media, states, "Trials are intended to generate data on how a drug works and you can’t generate that data if you don’t have participants in the study." Furthermore, the total campaign for a recent migraine study was 12 times less expensive than anticipated through traditional media outlets, further illustrating the cost-effectiveness of digital marketing strategies.

To effectively balance multiple clinical research endeavors, establishing clear timelines and milestones for each study is essential. Utilizing management tools significantly enhances the tracking of progress and resource distribution. Regular team meetings are vital for facilitating communication and proactively addressing potential bottlenecks. By allocating dedicated managers to each experiment, organizations can ensure careful oversight of all elements.
For example, a contract research organization (CRO) like bioaccess, which specializes in facilitating medical device clinical trials in Latin America, has shown the capability to accelerate regulatory approvals in just 6-8 weeks, compared to the typical 6-12 months in the US/EU. This efficiency enables the enrollment of treatment-naive cardiology or neurology cohorts 50% faster than Western sites, effectively overcoming patient recruitment challenges. A CRO that implemented a centralized management system experienced a 25% decrease in delays. Furthermore, initiatives are 2.5 times more successful when organized management practices are employed. This approach not only fosters teamwork among group members but also maintains clarity in schedules, ultimately enhancing overall productivity and efficacy in overseeing clinical studies. As highlighted by PMI, "Organizations that invest in proper management practices see better outcomes." By prioritizing organized project management practices, organizations can navigate the complexities of multiple tests more successfully, while remaining vigilant of common pitfalls such as scope creep and miscommunication that can derail progress.

To effectively utilize diverse patient groups, research studies must be designed with inclusivity and accessibility in mind. Collaborating with community organizations and advocacy groups significantly enhances awareness of research studies and their benefits. Furthermore, employing culturally aware personnel fosters trust and builds connections with underrepresented groups. It is also crucial to consider financial reimbursement for trial-related expenses, as this can eliminate barriers to participation and enhance diversity.
For instance, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a leading site for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only improves the recruitment process but also stimulates local economic growth through job creation and enhanced healthcare services. A recent study that focused on recruiting participants from diverse ethnic backgrounds illustrated that this inclusive approach not only improved the quality of data collected but also led to more equitable health outcomes. The DEPICT Act, which mandates the integration of diversity action plans in research designs, underscores the legislative support for these initiatives. By prioritizing diversity in medical studies, organizations can ensure their research is relevant and beneficial across all demographic segments, ultimately enhancing the overall impact of health investigations. As Dr. Marie Bernard emphasizes, "It’s essential to have diversity among those who design, recruit, and engage in clinical studies." This comprehensive approach can help mitigate common pitfalls in inclusive trial design, ensuring that the practices discussed are effectively implemented.

Mastering treatment and control in clinical trials is essential for producing reliable and impactful research outcomes. This conclusion underscores the necessity of adhering to regulatory compliance, implementing effective recruitment strategies, managing multiple projects efficiently, and leveraging diverse patient populations. Each of these elements plays a crucial role in enhancing the overall integrity and success of clinical studies.
Key arguments highlight that staying informed about regulatory requirements, such as those from the FDA and EMA, ensures that research teams maintain high standards of accountability and transparency. Furthermore, effective recruitment strategies that utilize digital marketing and community partnerships can significantly increase participant engagement and retention. Organized project management practices are vital for balancing various research timelines and mitigating common pitfalls. Lastly, prioritizing diversity within patient pools not only enriches data quality but also leads to more equitable health outcomes.
In light of these insights, it is evident that a comprehensive approach to clinical trial management can drive meaningful advancements in medical research. Embracing best practices for regulatory compliance, participant recruitment, project management, and inclusivity will enhance the credibility of research and ensure that clinical trials are relevant and beneficial to all demographic segments. Organizations are encouraged to adopt these strategies to elevate their research efforts and contribute to the ongoing evolution of healthcare solutions.
What is the importance of regulatory compliance in clinical trials?
Regulatory compliance is crucial in clinical trials to ensure adherence to guidelines from regulatory bodies like the FDA and EMA, which helps maintain the integrity and credibility of research studies.
What are the key requirements for regulatory compliance in clinical trials?
Key requirements include obtaining Institutional Review Board (IRB) approvals, following informed consent processes, and adhering to Good Clinical Practice (GCP) guidelines.
How can research teams stay informed about compliance responsibilities?
Regular training sessions for the research team are essential to ensure all members are aware of their compliance responsibilities.
What tools can help manage regulatory compliance effectively?
Compliance management software can streamline the tracking of regulatory documents and deadlines, reducing the risk of oversight.
What recent updates from the EMA are important for clinical trials?
Recent updates emphasize the importance of transparency and prompt reporting, including the requirement that all research studies must be registered in the Clinical Trials Information System (CTIS) before initiation.
What is the purpose of the Clinical Trials Regulation (CTR)?
The CTR aims to enhance public access to examination information and increase accountability among sponsors of clinical trials.
What does the FDAAA 801 Final Rule require?
The FDAAA 801 Final Rule requires the registration and reporting of clinical trial results.
What are the benefits of implementing a robust compliance strategy in clinical trials?
A robust compliance strategy, including regular audits and updates, can help organizations avoid costly delays and enhance the credibility of their research outcomes.
What consequences can arise from compliance failures in clinical trials?
Compliance failures can lead to substantial penalties and loss of funding, highlighting the critical need for rigorous compliance practices.