

The article underscores the critical necessity of mastering UDI machine-readable labels in Mexico, which is essential for compliance and success within the medical device industry.
Adherence to UDI regulations is paramount for ensuring patient safety, enhancing traceability, and improving operational efficiency.
Manufacturers must implement the specific labeling requirements mandated by COFEPRIS to avoid penalties and facilitate seamless market access.
This compliance not only safeguards patients but also positions manufacturers favorably in a competitive landscape.
The landscape of medical device regulation is evolving, with Unique Device Identification (UDI) emerging as a critical component in ensuring safety and efficiency within healthcare systems. This system not only enhances traceability and minimizes errors but also plays a vital role in regulatory compliance, particularly in markets like Mexico. As manufacturers navigate the complexities of UDI machine-readable labels, questions arise:
Exploring these challenges and solutions is essential for any organization aiming to thrive in the competitive medical device sector.
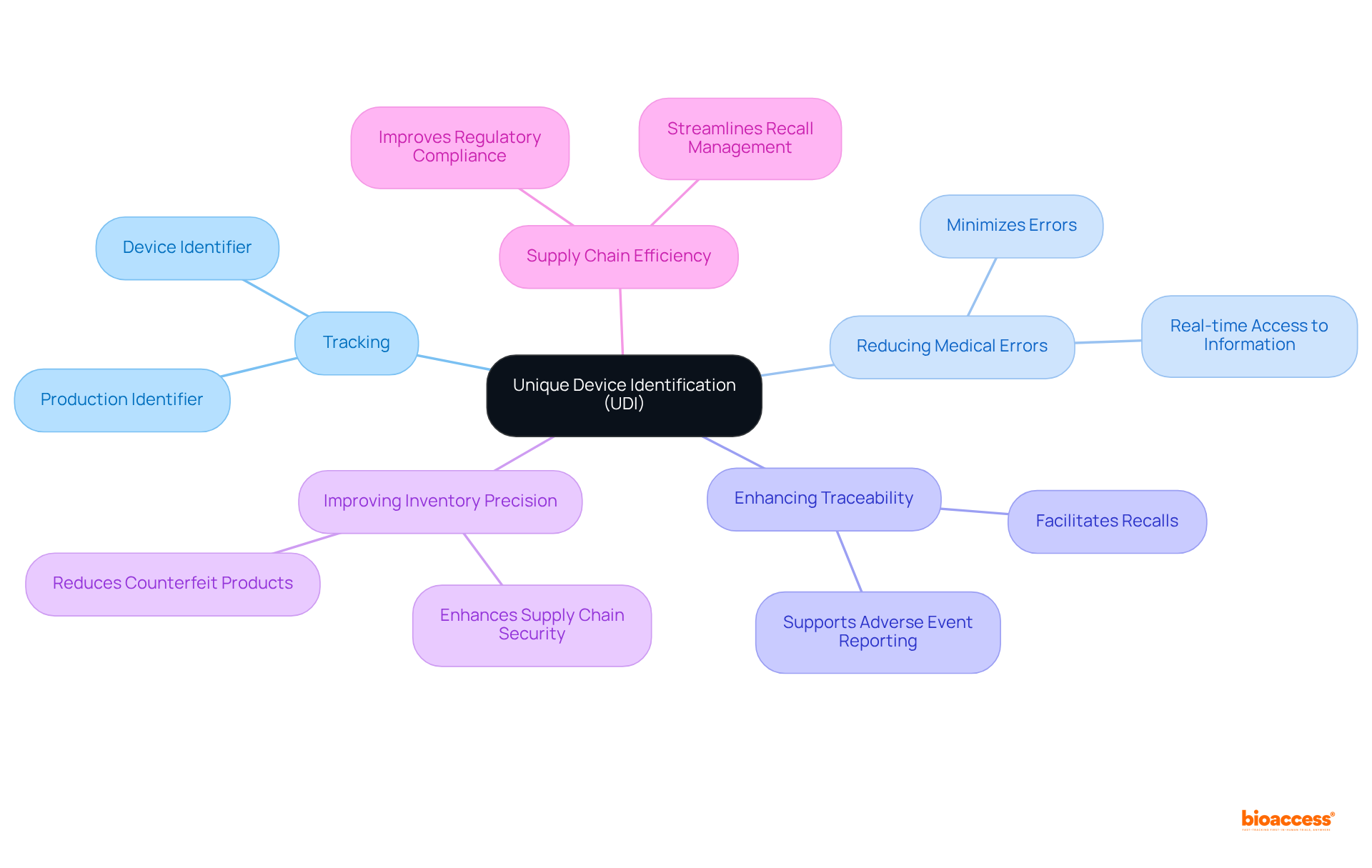
Unique Device Identification (UDI) stands as a pivotal system that assigns a distinct identifier to medical instruments, facilitating their tracking throughout the supply chain—from manufacturing to user application. This identifier is essential for healthcare professionals, enabling precise recognition and handling of equipment, which is critical for individual well-being.
The implementation of UDI significantly bolsters individual well-being by minimizing medical errors, enhancing traceability, and streamlining the recall process when necessary. For instance, the UDI system aids in the swift recognition of products during recalls, thereby reducing the time required for notifications and improving overall post-market security.
Furthermore, research has shown that UDI enhances inventory precision and diminishes the prevalence of fraudulent medical products, which are crucial elements in ensuring consumer safety. By adhering to UDI regulations, manufacturers not only comply with regulatory requirements but also contribute to improved healthcare outcomes, ultimately fostering a safer environment for patients.
The benefits of the UDI system extend beyond mere compliance; it enhances supply chain efficiency and facilitates better recall management, rendering it an indispensable tool in modern healthcare.
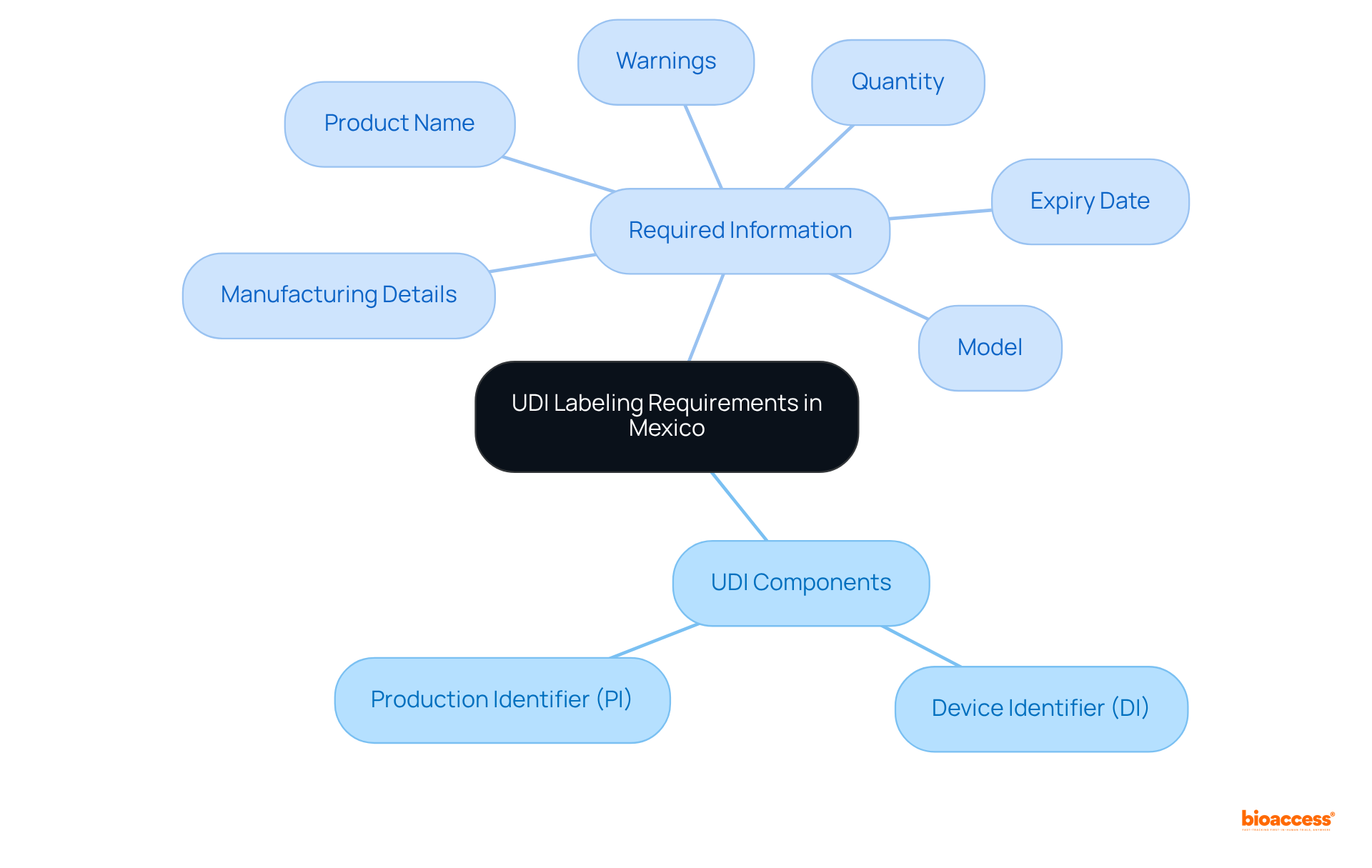
In Mexico, the UDI machine-readable label requirements are overseen by COFEPRIS, the regulatory authority responsible for health products. It is imperative for manufacturers to label their medical products with a UDI machine-readable label Mexico that includes both human-readable and machine-readable formats, such as barcodes. A UDI comprises two primary elements:
Essential information on the label must encompass:
Adherence to these regulations, such as the UDI machine-readable label Mexico, is not optional; it is required for all medical products sold in Mexico. Non-compliance can lead to severe penalties and hinder market access. Therefore, a thorough understanding of these requirements, including the recent updates from NOM-137-SSA1-2024, is vital for successful product registration and commercialization within the Mexican healthcare market.
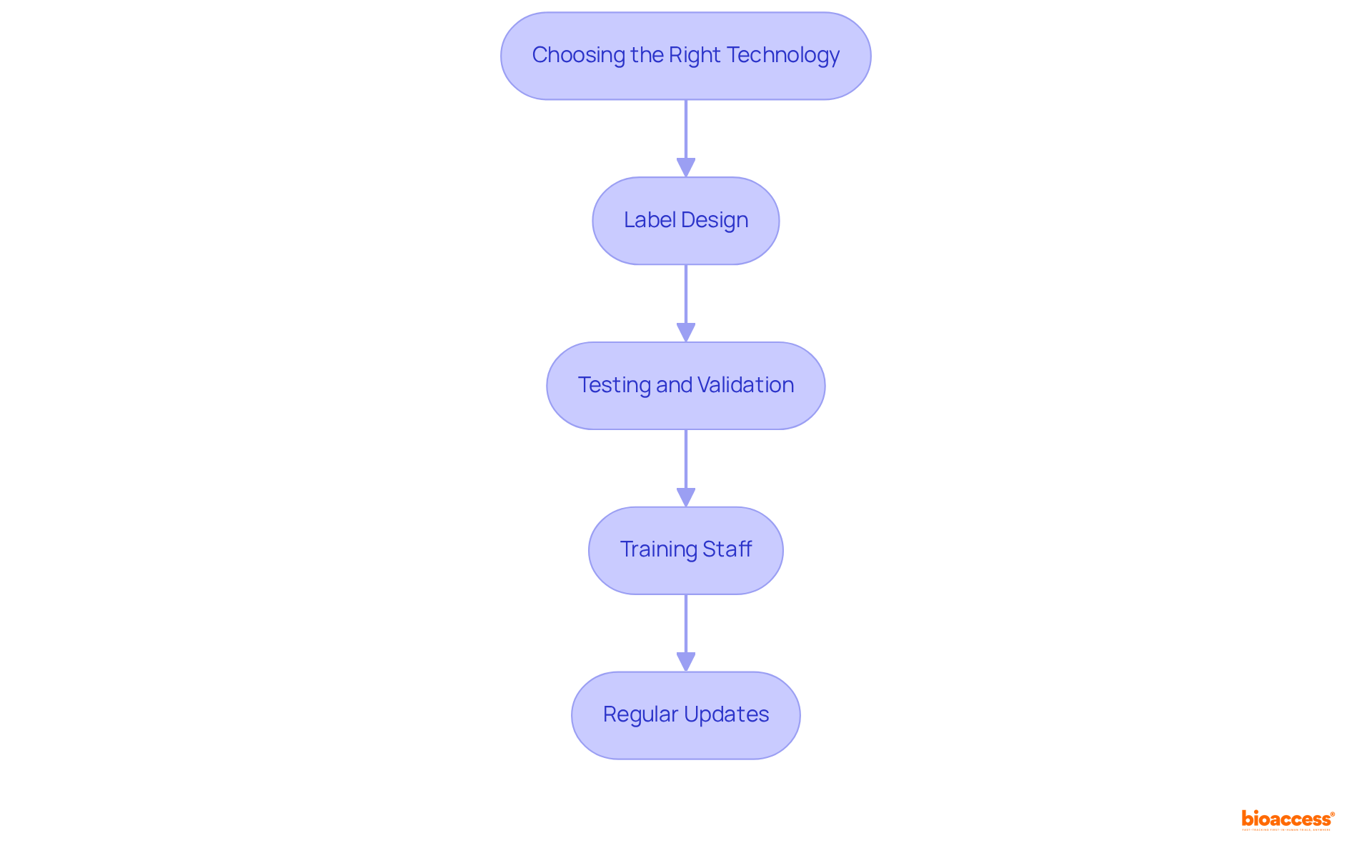
To implement the UDI machine-readable label Mexico, manufacturers must leverage various technologies, including barcodes and RFID tags. Understanding best practices for UDI labeling is crucial for success:
By adhering to these best practices, manufacturers can enhance their success rates in implementing the UDI machine-readable label Mexico, ultimately improving individual well-being and device traceability within the healthcare system.
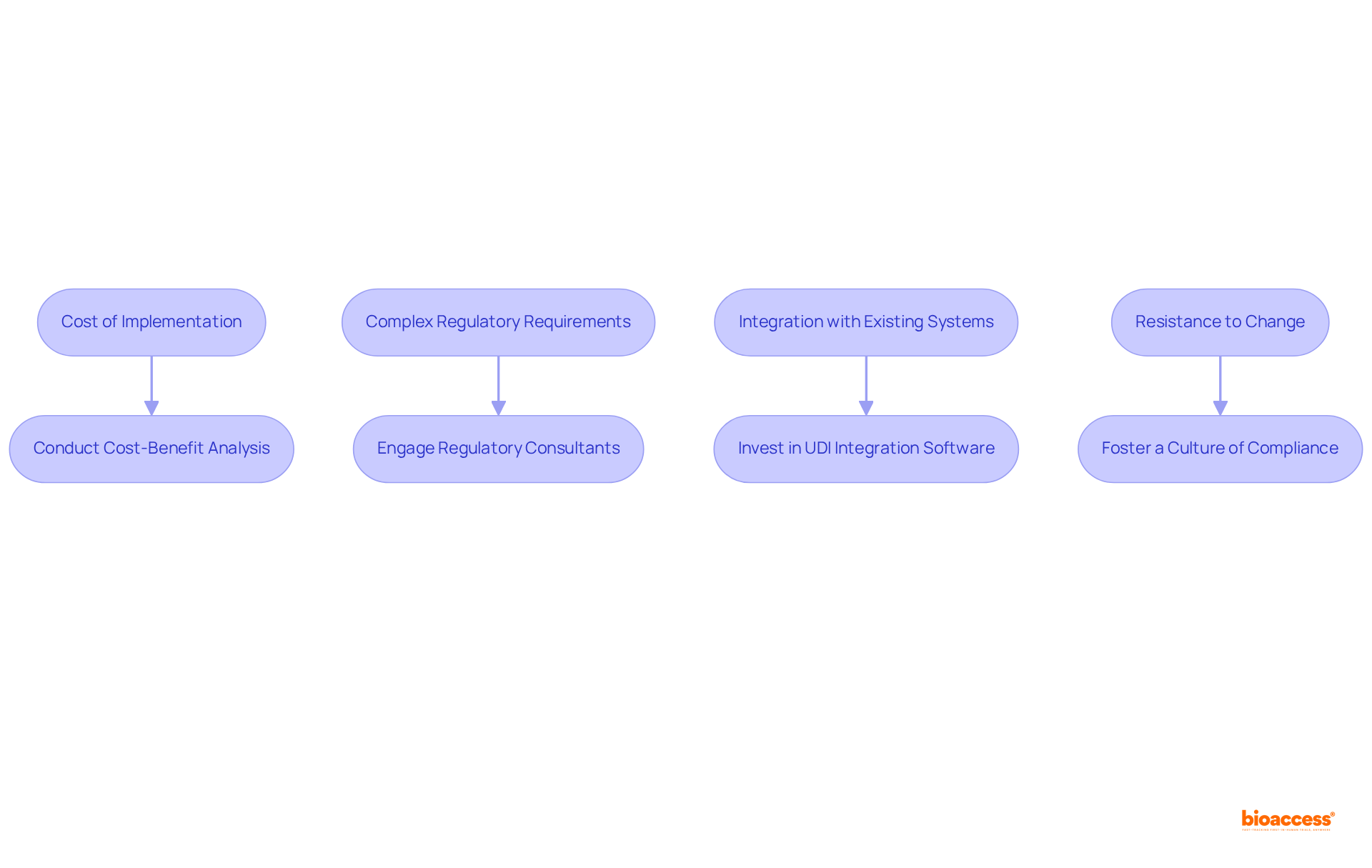
Adopting UDI labeling presents several challenges that manufacturers must navigate effectively.
The cost of implementation can be significant, as high initial expenses for technology and training may arise. Conducting a cost-benefit analysis is essential to justify this investment and explore available funding options.
The complex regulatory requirements can be daunting, making compliance a critical concern. Engaging with regulatory consultants or legal experts can provide the necessary guidance to ensure adherence to these regulations.
Another challenge is the integration with existing systems, as current inventory and tracking solutions may not support UDI. Investing in software that facilitates UDI integration, along with comprehensive staff training, is a vital step toward overcoming this hurdle.
Lastly, resistance to change among employees can hinder the adoption of new processes. It is crucial to foster a culture of compliance by emphasizing the benefits of UDI, particularly in enhancing patient safety and operational efficiency.
The implementation of Unique Device Identification (UDI) stands as a cornerstone for ensuring safety and efficiency within the medical device industry. By assigning a specific identifier to each medical product, UDI not only facilitates accurate tracking and management throughout the supply chain but also significantly enhances patient safety by reducing medical errors and improving recall processes.
This article emphasizes several key aspects of UDI, including:
Compliance with COFEPRIS regulations is crucial, as is the necessity of robust label design, alongside ongoing staff training to effectively navigate the complexities of UDI adoption. Furthermore, the discussion on overcoming challenges such as cost, integration with existing systems, and employee resistance underscores the multifaceted approach required for successful UDI implementation.
In conclusion, embracing UDI transcends mere regulatory obligation; it represents a vital step toward enhancing healthcare outcomes and ensuring patient safety. As manufacturers and researchers navigate the complexities of UDI labeling, a commitment to best practices and continuous improvement will be essential. By prioritizing UDI compliance, stakeholders can contribute to a safer healthcare environment and foster trust in medical products, ultimately benefiting patients and healthcare providers alike.
What is Unique Device Identification (UDI)?
Unique Device Identification (UDI) is a system that assigns a distinct identifier to medical devices, allowing for their tracking throughout the supply chain, from manufacturing to user application.
Why is UDI important in healthcare?
UDI is important because it enables precise recognition and handling of medical equipment, which is critical for individual well-being and helps minimize medical errors.
How does UDI improve patient safety?
UDI improves patient safety by enhancing traceability, reducing the prevalence of fraudulent medical products, and streamlining the recall process, which aids in the swift recognition of products during recalls.
What are the benefits of implementing UDI for manufacturers?
Implementing UDI helps manufacturers comply with regulatory requirements, enhances inventory precision, improves healthcare outcomes, and contributes to a safer environment for patients.
How does UDI affect supply chain efficiency?
UDI enhances supply chain efficiency by improving inventory management and facilitating better recall management, making it an indispensable tool in modern healthcare.