


This article delves into mastering VHH nanobody strategies, essential for achieving success in clinical research. It emphasizes their unique properties, production methods, and diverse applications in diagnostics and therapeutics. By detailing the stability, low immunogenicity, and engineering capabilities of VHH nanobodies, the article illustrates their significance as valuable tools in medical science. Their successful use in clinical trials and therapeutic approvals serves as compelling evidence of their impact. Understanding these strategies is crucial for advancing clinical research and addressing the evolving challenges within the Medtech landscape.
VHH nanobodies, derived from camelids, are transforming the landscape of clinical research with their unique properties, including remarkable stability and the capacity to target elusive epitopes. As the demand for innovative therapeutic and diagnostic solutions escalates, comprehending the strategies for effective VHH nanobody generation and production becomes imperative. However, the journey to harness their full potential presents challenges—what are the key factors that can ensure success in this rapidly evolving field?
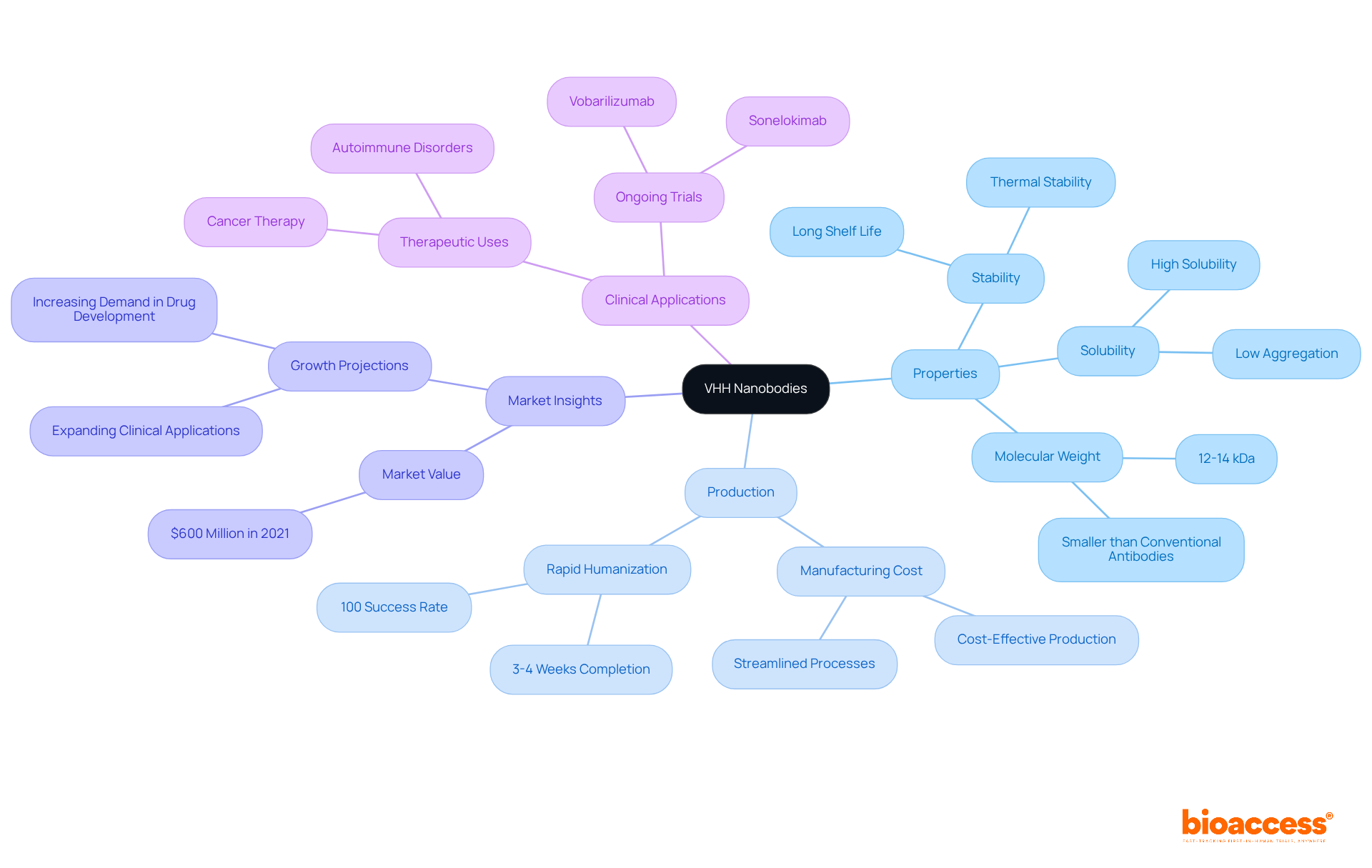
VHH single-domain antibodies, derived from camelids, exhibit remarkable properties, including exceptional stability and solubility. Their small molecular weight, typically around 12-14 kDa, facilitates superior tissue penetration and enables binding to challenging targets, including hidden epitopes that conventional antibodies often cannot access. Significantly, VHH antibodies can be preserved for extended durations at temperatures exceeding 90°C, further enhancing their stability for medical applications. This unique structural advantage positions VHH proteins as ideal candidates for both therapeutic and diagnostic uses.
The production of VHH antibodies in microbial systems streamlines the manufacturing process, significantly reducing costs and time. For instance, Sino Biological has demonstrated a 100% success rate in humanizing small antibodies, accomplishing this in as little as 3-4 weeks. This rapid humanization process not only enhances their therapeutic potential but also preserves low immunogenicity, a crucial factor for clinical application. Furthermore, VHH fragments exhibit more than 80% sequence similarity with human heavy-chain antibodies, reinforcing their low immunogenicity and suitability for human use.
Recent studies indicate that the global market for VHH antibodies was valued at approximately $600 million in 2021, with projections for substantial growth driven by increasing demand in drug development and diagnostics. The stability and solubility of VHH fragments have been validated through various case studies, confirming their effectiveness in diverse applications, including cancer therapy and molecular imaging. Notably, these small antibodies have received several therapeutic approvals for addressing cancers and autoimmune disorders, underscoring their clinical significance.
As researchers continue to explore the benefits of VHH antibodies in clinical research, their ability to be engineered for specific functions further enhances their appeal. Ongoing clinical trials for Vobarilizumab and sonelokimab underscore the dynamic research landscape surrounding VHH nanobodies. However, it is essential to acknowledge potential limitations, such as low serum persistence due to their small size, which may impact their treatment efficacy.
The ongoing advancements in VHH technology highlight their potential to transform treatment strategies, making them a valuable asset in the biopharmaceutical landscape. Expert insights suggest that the unique characteristics of VHH fragments, combined with their high purity attained through techniques like immobilized metal affinity chromatography (IMAC), position them as promising tools in both therapeutic and diagnostic applications.
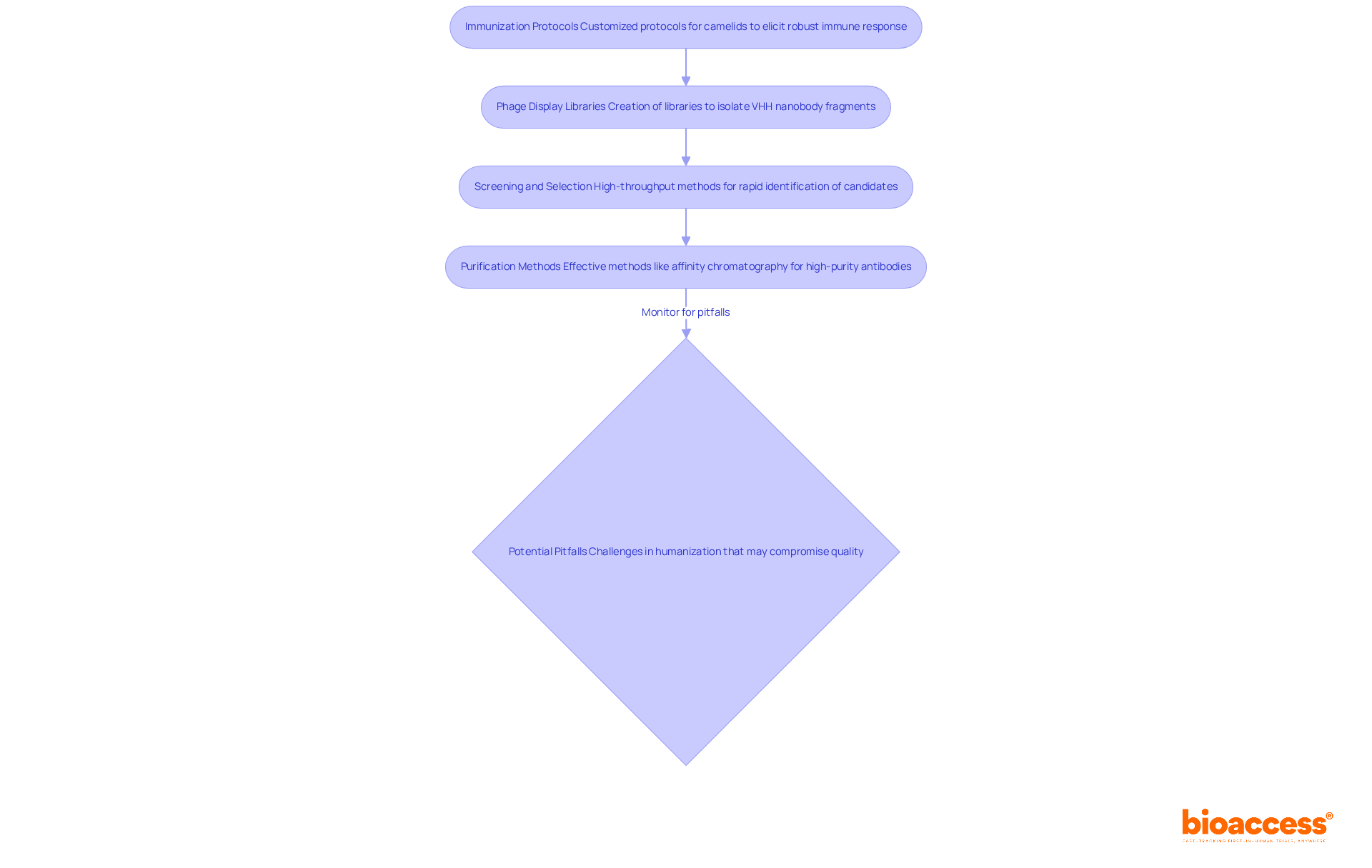
To effectively generate and produce vhh nanobody antibodies, researchers must adopt a systematic approach that encompasses several key strategies.
Immunization Protocols: Implementing customized immunization protocols for camelids is essential to elicit a robust immune response, thereby ensuring a diverse range of antibodies. Notably, the success rate of identifying small antibodies from immune libraries approaches 100% when a properly folded protein is utilized as an immunogen.
Phage Display Libraries: Creating various phage display libraries has proven to be highly successful in isolating VHH nanobody fragments, allowing for the screening of high-affinity candidates.
Screening and Selection: Employing high-throughput screening methods enables rapid identification of promising nanobody candidates, significantly enhancing efficiency in the selection process. Selecting suitable expression systems, such as E. coli or yeast, facilitates the efficient production of vhh nanobody fragments at scale.
Purification Methods: Finally, utilizing effective purification methods, including affinity chromatography, ensures the separation of high-purity antibodies, making them appropriate for clinical use. It is crucial to remain vigilant about potential pitfalls, such as challenges in humanization that may compromise quality.
By implementing these strategies, researchers can substantially improve the yield and quality of vhh nanobody antibodies, ultimately leading to more effective clinical applications.
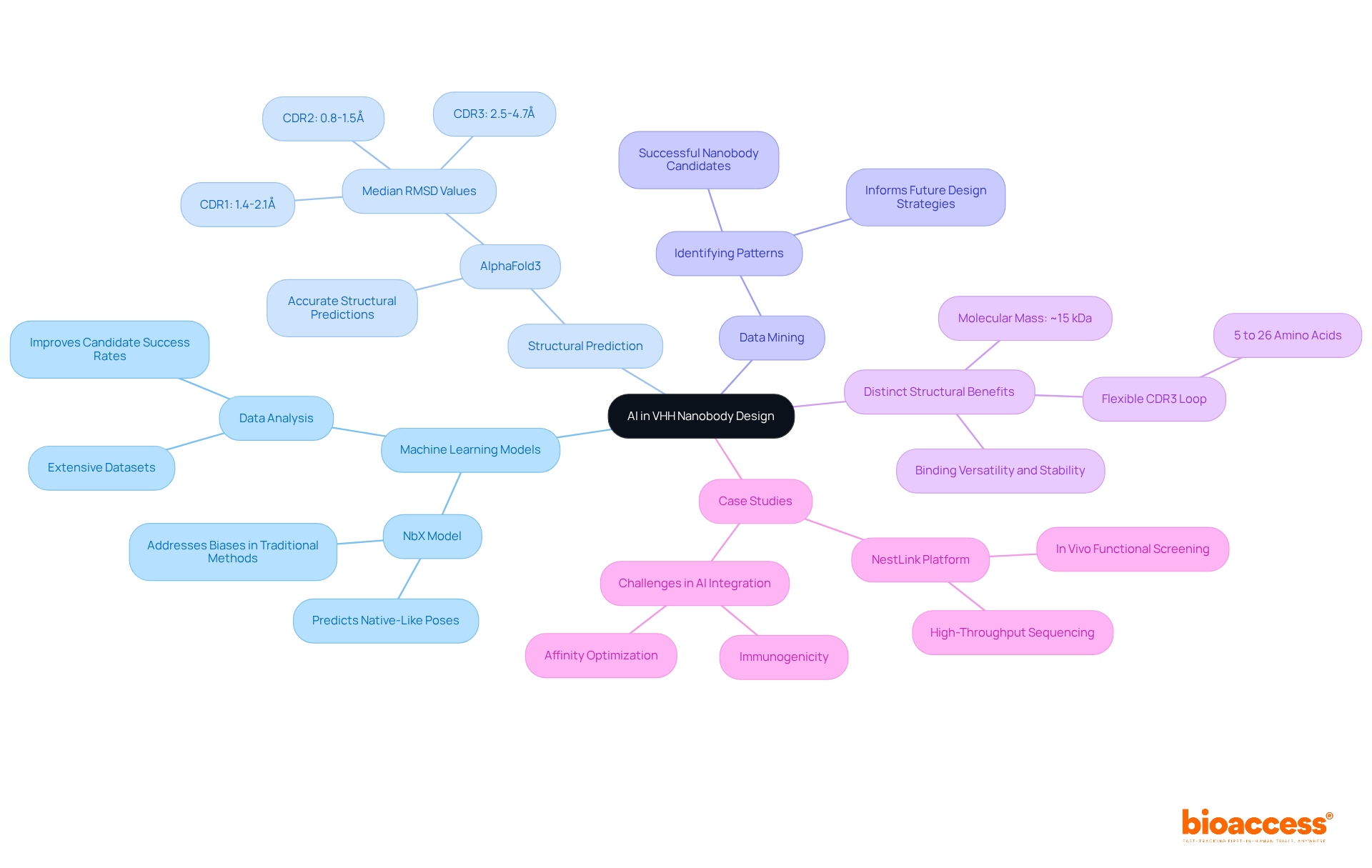
Artificial Intelligence (AI) is revolutionizing the design and optimization of vhh nanobodies by offering significant advantages in predicting binding affinities and enhancing their structural properties. Key applications include:
Machine Learning Models: Advanced machine learning models analyze extensive datasets to predict the success of new nanobody designs, significantly improving the likelihood of effective candidates. For instance, models like NbX utilize machine learning to forecast native-like poses, addressing biases in traditional methods.
Structural Prediction: AI tools, such as AlphaFold3, provide accurate structural predictions that guide modifications to enhance stability and binding affinity. These tools have demonstrated high precision in predicting CDR structures, with median RMSD values of 1.4-2.1Å for CDR1, 0.8-1.5Å for CDR2, and 2.5-4.7Å for CDR3, which is crucial for optimizing nanobody functionality.
Data Mining: Data mining techniques are employed to identify patterns in successful nanobody candidates, informing future design strategies. This approach allows researchers to leverage historical data to refine their designs, enhancing the efficiency of the development process.
Distinct Structural Benefits: Nanobodies, with a molecular mass of around 15 kDa and a flexible CDR3 loop varying from 5 to 26 amino acids, display distinct structural characteristics that improve their binding versatility and stability, rendering them especially appropriate for use in drug conjugates.
Case Studies: Platforms such as NestLink merge in vivo functional screening with high-throughput sequencing, improving the discovery and optimization of small antibodies. Additionally, challenges such as immunogenicity and the need for affinity optimization are critical considerations in the integration of AI into nanobody design.
Integrating AI into the vhh nanobody development process not only accelerates optimization but also reduces the time and resources required, leading to faster advancements in clinical research. As AI progresses, its uses in nanobody engineering are anticipated to grow, creating opportunities for groundbreaking treatment solutions.
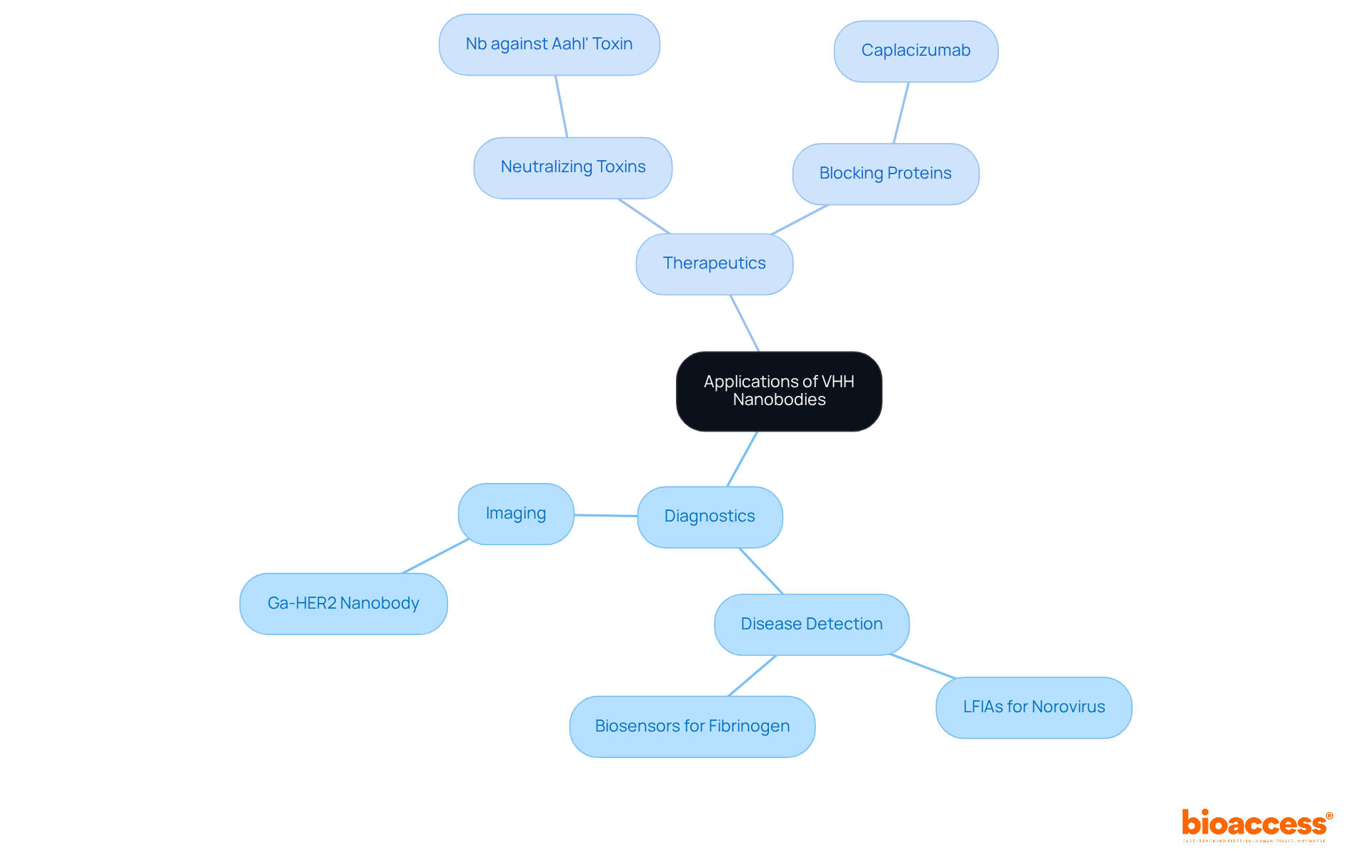
VHH nanobody fragments are emerging as versatile tools in both diagnostics and therapeutics, showcasing the potential of VHH nanobody to revolutionize medical applications. These tiny antibodies function as highly specific probes for imaging and detecting diseases, including cancer and infectious illnesses. For instance, VHH fragments can be conjugated with imaging agents, significantly enhancing tumor visualization in imaging studies. Their ability to penetrate tissues more effectively than conventional antibodies allows for improved detection rates and specificity. The domain of small antibodies has expanded rapidly since their discovery a little more than thirty years ago, highlighting their rising importance in medical science.
In the medical field, VHH nanobody fragments can be designed to neutralize toxins or block disease-causing proteins, offering a new method for treatment. Their small size, typically ranging from 5 to 200 nm, and remarkable stability under various conditions make them suitable for diverse delivery methods, including oral and inhalation routes. The first nanobody-based drug, Caplacizumab, is a bivalent nanobody approved by the EMA and FDA for treating thrombotic thrombocytopenic purpura (TTP). Clinical studies have demonstrated that patients receiving Caplacizumab exhibited quicker platelet count normalization compared to placebo, marking a significant milestone in nanobody treatment.
Recent studies have highlighted the success rates of VHH nanobody in cancer detection and treatment. For example, a Ga-HER2 nanobody developed for PET imaging in breast cancer has shown promising results in detecting HER2 expression, with rapid renal clearance and high uptake in metastatic lesions. This underscores the potential of the VHH nanobody molecules to improve diagnostic precision and therapeutic outcomes, especially in cancers where EGFR gene amplifications and activating mutations are found in up to 70% of glioblastomas.
As research continues to explore these applications, the VHH nanobody is poised to contribute significantly to advancements in medical science, offering innovative solutions that can enhance patient outcomes across various diseases.
VHH nanobodies signify a revolutionary leap in clinical research, showcasing distinct properties that set them apart from conventional antibodies. Their remarkable stability, solubility, and capability to target difficult epitopes render them essential tools in therapeutic and diagnostic settings. As the biopharmaceutical landscape progresses, comprehending and leveraging the potential of VHH nanobodies is crucial for fostering innovation and enhancing patient outcomes.
This article has delved into critical insights regarding the generation, production, and applications of VHH nanobodies. Key strategies, including:
are vital for optimizing these small antibodies for clinical application. Furthermore, the increasing market demand and successful case studies highlight the promising future of VHH nanobodies in tackling complex diseases, particularly in cancer therapy and molecular imaging.
Given these advancements, the importance of VHH nanobodies is undeniable. As research continues to reveal their capabilities, stakeholders in the medical and biopharmaceutical sectors are urged to invest in the development and utilization of these innovative tools. Embracing the distinctive features of VHH nanobodies could lead to groundbreaking treatment strategies and diagnostic methods that significantly improve patient care and shape the future of medicine.
What are VHH nanobodies?
VHH nanobodies are single-domain antibodies derived from camelids, known for their exceptional stability, solubility, and small molecular weight, which allows them to penetrate tissues effectively and bind to challenging targets.
What is the typical molecular weight of VHH antibodies?
The typical molecular weight of VHH antibodies is around 12-14 kDa.
How do VHH antibodies compare to conventional antibodies in terms of stability?
VHH antibodies can be preserved for extended durations at temperatures exceeding 90°C, showcasing superior stability compared to conventional antibodies.
What advantages do VHH antibodies offer for therapeutic and diagnostic applications?
Their unique structural properties, such as stability, solubility, and ability to bind to hidden epitopes, make VHH antibodies ideal candidates for both therapeutic and diagnostic uses.
How are VHH antibodies produced, and what benefits does this offer?
VHH antibodies are produced in microbial systems, which streamlines the manufacturing process, significantly reducing costs and time.
What is the success rate of humanizing small antibodies, and how long does it typically take?
Sino Biological has demonstrated a 100% success rate in humanizing small antibodies, accomplishing this in as little as 3-4 weeks.
Why is low immunogenicity important for VHH antibodies?
Low immunogenicity is crucial for clinical application as it reduces the likelihood of adverse immune reactions in patients.
What was the estimated global market value for VHH antibodies in 2021?
The global market for VHH antibodies was valued at approximately $600 million in 2021.
What applications have VHH antibodies been validated for?
VHH antibodies have been validated for use in cancer therapy and molecular imaging, among other applications.
What ongoing research is being conducted on VHH antibodies?
Ongoing clinical trials for Vobarilizumab and sonelokimab highlight the dynamic research landscape surrounding VHH nanobodies.
What are some limitations of VHH antibodies?
One potential limitation is their low serum persistence due to their small size, which may impact treatment efficacy.
How do advancements in VHH technology impact the biopharmaceutical landscape?
Advancements in VHH technology highlight their potential to transform treatment strategies, making them valuable assets in the biopharmaceutical industry.