


The article delineates the objectives, ethical considerations, and outcomes inherent to Phase 2 clinical trials, which are pivotal for assessing the effectiveness and safety of new therapies once initial safety has been validated. It underscores that these trials not only guide decisions regarding progression to Phase 3 studies but also comply with stringent regulatory and ethical standards designed to safeguard participants and ensure the integrity of research findings.
Understanding the intricacies of clinical trial phase 2 is essential for anyone involved in drug development and medical research. This phase serves as a critical juncture, where the effectiveness of a treatment is rigorously tested after initial safety assessments, paving the way for informed decisions about its future.
As researchers navigate the complex landscape of objectives, methodologies, and ethical considerations, the stakes are high—successful outcomes can propel a drug into the next phase of trials, while failures may halt its journey.
What challenges and opportunities lie within this pivotal stage, and how do they shape the future of healthcare? This exploration not only underscores the significance of phase 2 trials but also invites stakeholders to consider their role in advancing medical innovation.
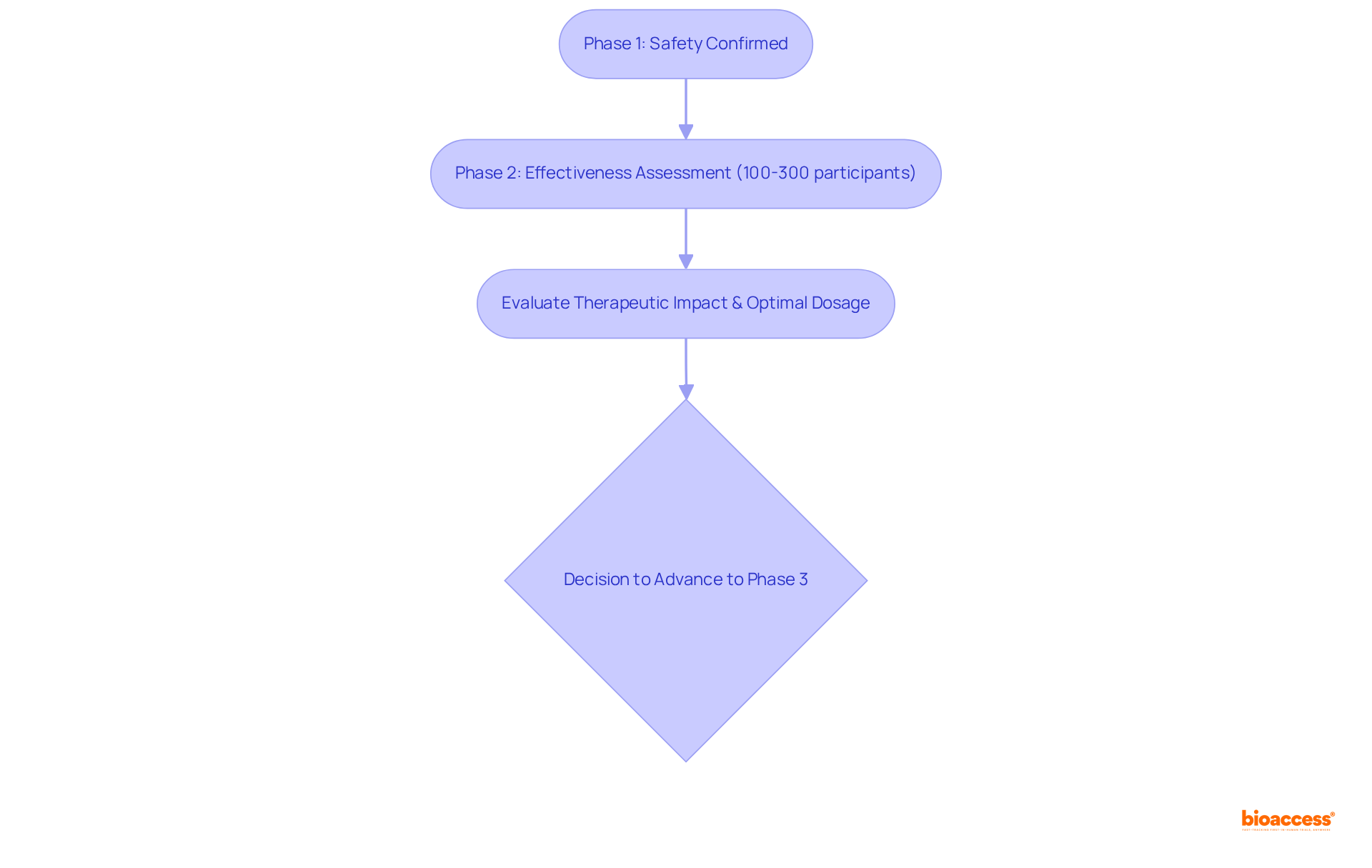
Clinical trial phase 2 studies are crucial for assessing the effectiveness of a medication or therapy after the initial safety has been confirmed in Stage 1 studies. These studies typically involve a larger cohort, ranging from 100 to 300 individuals, and are vital for determining whether the intervention achieves the desired therapeutic impact.
The significance of clinical trial phase 2 studies lies in their ability to provide preliminary insights into the intervention's performance, which is essential for making informed decisions about advancing to Stage 3 studies. Additionally, this phase helps establish optimal dosages and further evaluates the regimen's risk profile within a more diverse patient population.
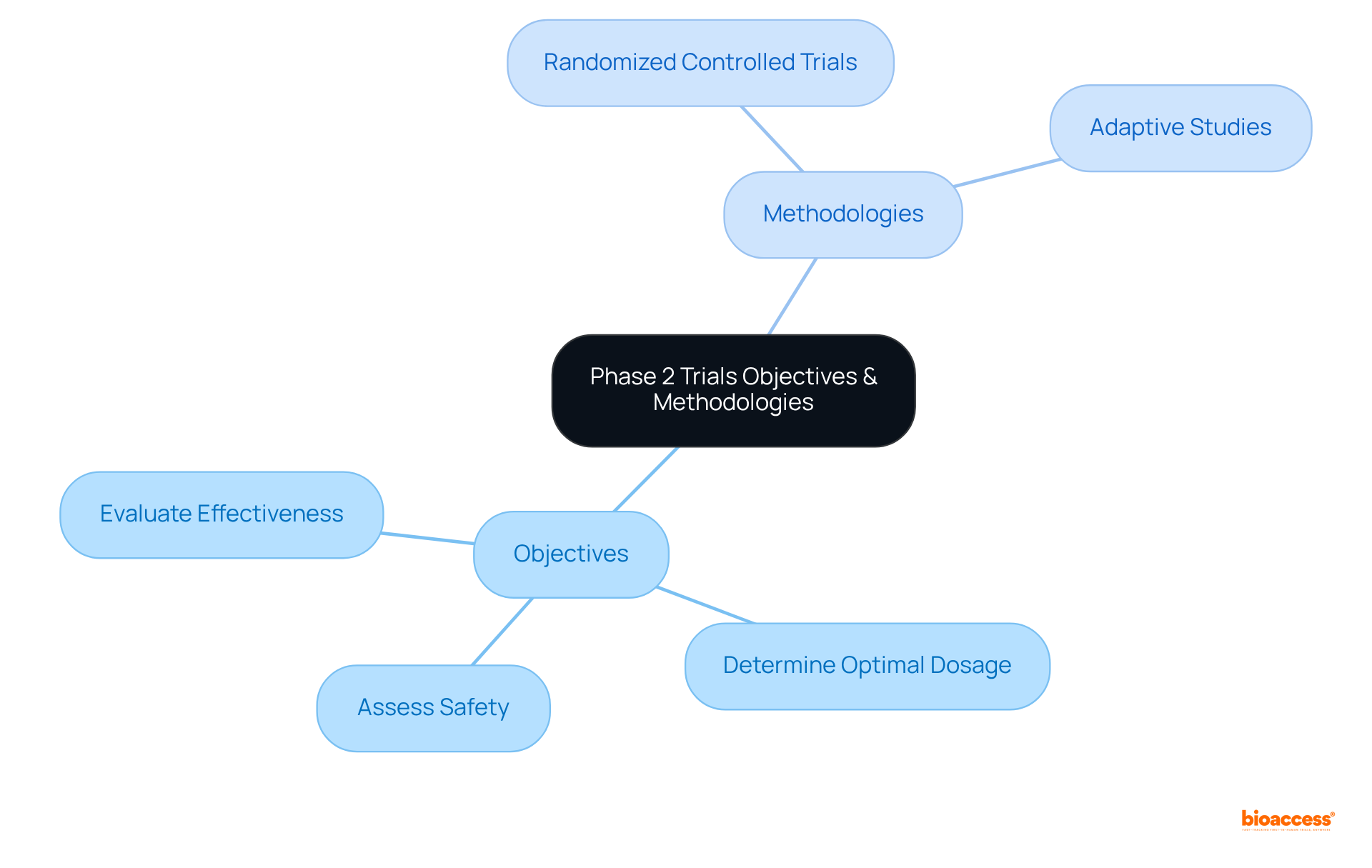
The primary objectives of clinical trial phase 2 studies are to:
Methodologies may vary; however, common designs include:
These approaches are essential for generating robust data that can support regulatory submissions and guide future research endeavors. Additionally, in clinical trial phase 2, trials often incorporate biomarkers and patient-reported outcomes, providing a comprehensive understanding of the treatment's effects.
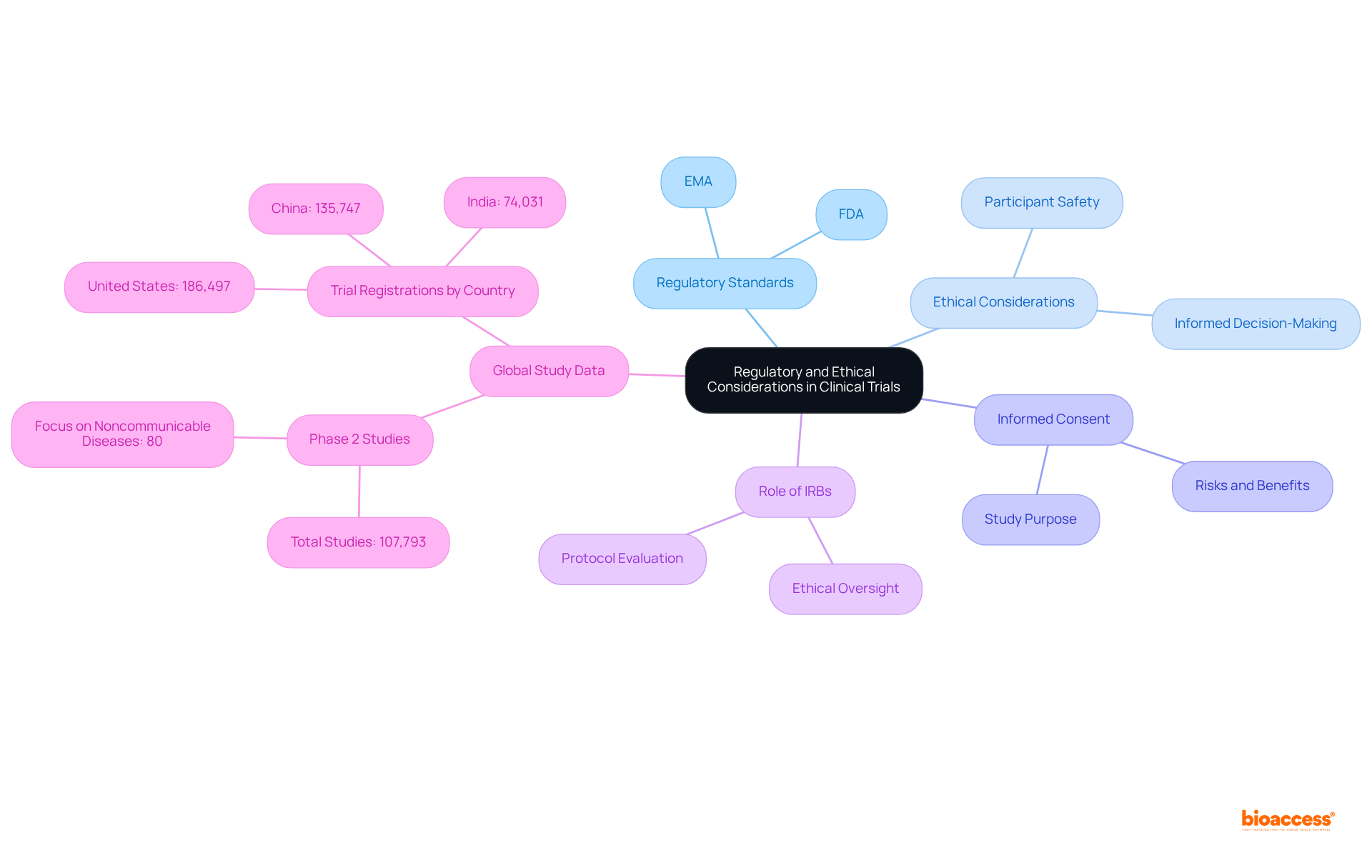
The regulatory standards established by organizations such as the FDA and EMA must be strictly adhered to in clinical trial phase 2 studies. This includes securing necessary approvals before initiating the experiment and ensuring that all ethical aspects are thoroughly considered. Informed consent is a vital component, requiring that participants are fully aware of the study's purpose, procedures, risks, and benefits. Furthermore, Institutional Review Boards (IRBs) play a crucial role in evaluating the study protocol to ensure participant safety and ethical conduct. Compliance with these regulations not only safeguards participants but also enhances the credibility of the research findings.
Significantly, information from clinical studies registered globally from 1999 to June 2024 reveals that Phase 2 studies constitute the largest segment, with 107,793 identified. The United States recorded the highest total count of tests registered during this period, with 186,497 tests, underscoring the critical importance of ethical oversight in this phase. Moreover, the analysis indicates that 80% of classified studies focus on noncommunicable diseases, emphasizing the ethical necessity to ensure participant safety and informed decision-making in research addressing major health issues.
In this context, bioaccess® plays a vital role in facilitating clinical studies across Latin America, the Balkans, and Australia, significantly contributing to the ethical and regulatory landscape of clinical trial phase 2.
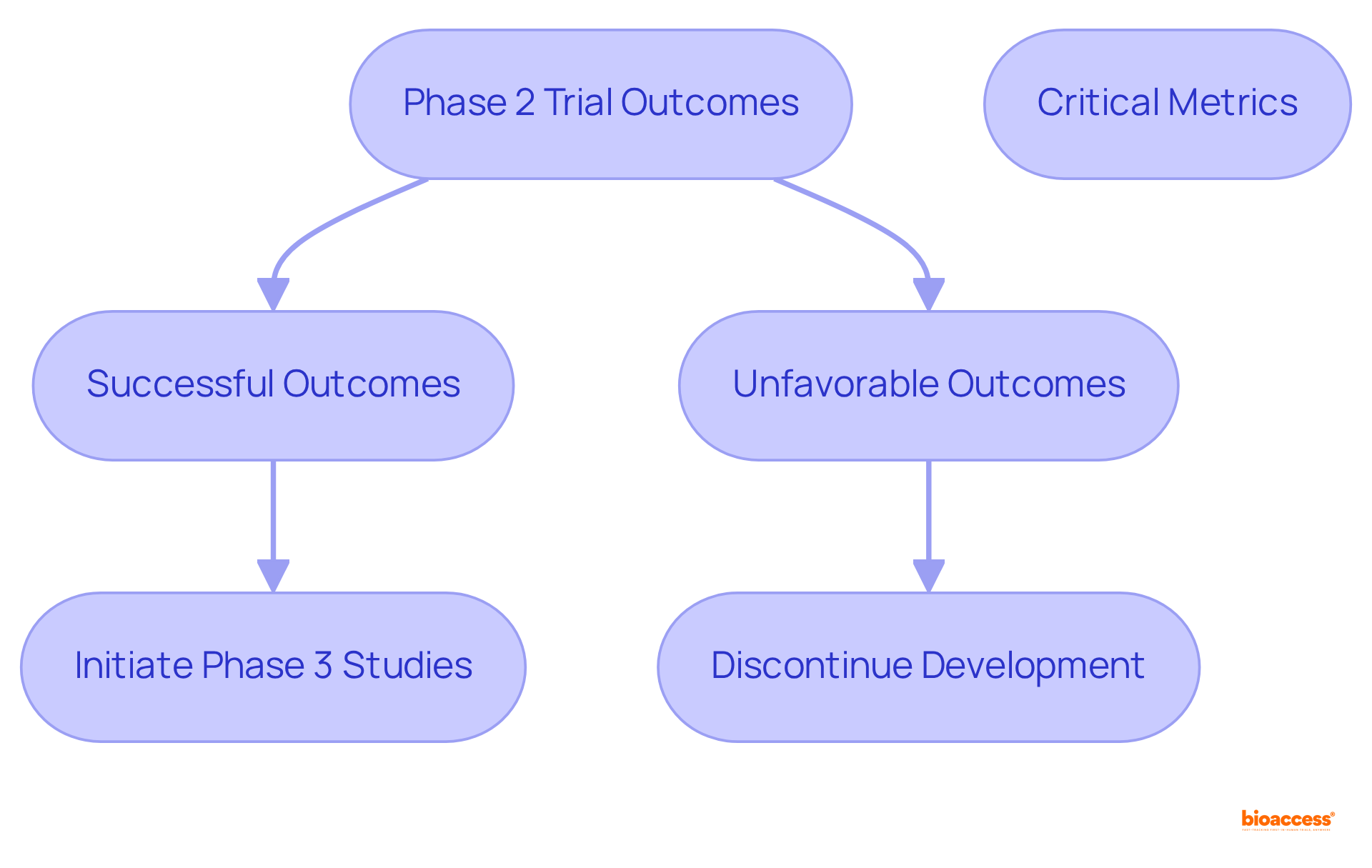
The results of Stage 2 studies are pivotal in determining the next steps in the clinical development process. Successful outcomes can lead to the initiation of Phase 3 studies, where larger populations are assessed to confirm effectiveness and monitor adverse reactions. Conversely, unfavorable results may prompt sponsors to discontinue further development.
Critical metrics for evaluating outcomes include:
The ramifications of these findings extend well beyond the trial itself, impacting regulatory decisions, market access strategies, and ultimately shaping patient care practices.
Clinical trial phase 2 studies serve as a critical bridge between initial safety assessments and the larger-scale investigations of phase 3 trials. These studies not only evaluate the effectiveness of new therapies but also help determine optimal dosages and safety profiles, all while adhering to stringent regulatory and ethical standards. The insights gained from phase 2 trials are essential for making informed decisions about the future of drug development and ultimately improving patient care.
Throughout this article, key aspects of phase 2 trials have been explored, including their primary objectives, methodologies, and the importance of regulatory and ethical considerations. The discussion has highlighted how randomized controlled trials and adaptive study designs contribute to robust data collection, while the role of informed consent and Institutional Review Boards ensures participant safety and ethical conduct. The significant number of phase 2 studies registered globally underscores their importance in the clinical research landscape.
Ultimately, the outcomes of phase 2 trials have far-reaching implications that extend beyond the research setting. Successful results can propel a therapy into phase 3 studies, influencing regulatory decisions and shaping market access strategies. As the landscape of clinical research continues to evolve, the importance of mastering phase 2 trials cannot be overstated. Engaging with this critical phase not only enhances the credibility of research findings but also paves the way for innovations that can transform patient care.
What are Phase 2 clinical trials?
Phase 2 clinical trials are studies that assess the effectiveness of a medication or therapy after initial safety has been confirmed in Phase 1 studies.
How many participants are typically involved in Phase 2 clinical trials?
Phase 2 clinical trials typically involve a larger cohort of participants, ranging from 100 to 300 individuals.
Why are Phase 2 clinical trials important?
Phase 2 clinical trials are important because they provide preliminary insights into the intervention's performance, helping to determine if it achieves the desired therapeutic impact and informing decisions about advancing to Phase 3 studies.
What additional information do Phase 2 trials provide?
Phase 2 trials help establish optimal dosages and further evaluate the regimen's risk profile within a more diverse patient population.