


The article emphasizes the mastery of European Medical Device Regulation (MDR) as a pivotal factor for attaining clinical success within the healthcare market. It underscores the necessity for manufacturers to comprehend and comply with the MDR, which encompasses stringent compliance processes and the significance of technical documentation. This understanding is essential for ensuring patient safety, securing market access, and adeptly navigating the complexities of regulatory requirements.
Navigating the intricate landscape of medical device regulation in Europe transcends mere compliance; it serves as a pivotal determinant of clinical success. The European Medical Device Regulation (MDR) imposes stringent requirements that manufacturers must fulfill to guarantee patient safety and product efficacy, necessitating comprehensive documentation and robust clinical evidence.
As the regulatory environment continues to evolve, manufacturers confront the pressing challenge of adapting to new standards while simultaneously striving to enhance patient outcomes.
How can Medtech innovators adeptly master these regulations to not only achieve compliance but also propel clinical excellence?
The European Medical Device Regulation (MDR) establishes a robust framework that governs the safety and performance of healthcare instruments within the European Union (EU). Central to this regulation is a steadfast commitment to patient safety and product efficacy, requiring manufacturers to provide comprehensive evidence of compliance through rigorous testing and meticulous documentation. Key elements of the European Medical Device Regulation include:
Navigating this complex compliance landscape is essential for ensuring that healthcare products meet the stringent criteria necessary for market access. A deep understanding of the European Medical Device Regulation not only aids in compliance but also equips companies to adapt to evolving regulatory requirements, which is crucial for achieving clinical success in Europe. As the landscape continues to evolve, remaining informed about patient safety statistics and the implications of the European Medical Device Regulation will empower Medtech innovators to refine their strategies and ultimately enhance patient outcomes.
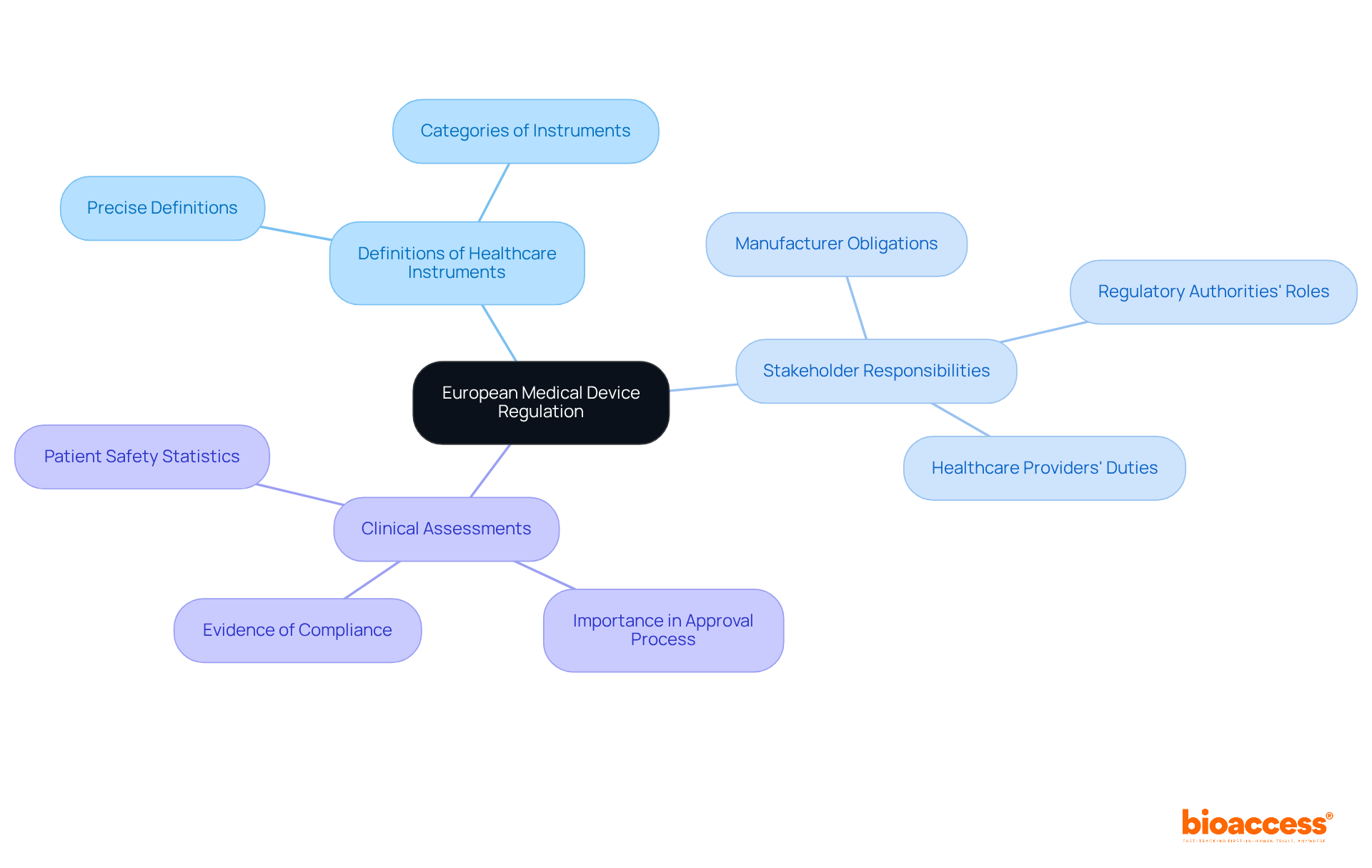
The primary legislation governing medical instruments in Europe is anchored in the Medical Devices Regulation (EU) 2017/745 and the In Vitro Diagnostic Medical Instruments Regulation (EU) 2017/746. These regulations supersede the previous Medical Devices Directive (MDD) and In Vitro Diagnostic Devices Directive (IVDD), establishing a robust framework for classification, conformity assessment, and post-market surveillance. For manufacturers, adherence to these regulations is paramount, as they delineate the essential steps for market access, including the requirement for a Notified Body's involvement for certain product categories.
As of 2023, the adherence rates for medical equipment under these EU regulations have shown significant improvement, with 33% of certified products classified as Class IIb. This classification indicates a medium risk level, necessitating thorough clinical evaluation and ongoing post-market surveillance. Furthermore, the In Vitro Diagnostic Medical Devices Regulation (EU) 2017/746 imposes additional requirements on manufacturers, underscoring the necessity for rigorous clinical evidence and continuous monitoring of device performance.
Producers navigating these regulations face challenges, particularly in managing compliance intricacies and maintaining relationships with Notified Bodies, often complicated by capacity constraints. To mitigate these challenges, manufacturers can leverage comprehensive clinical trial management services, such as those provided by bioaccess. These services include:
By harnessing these capabilities, manufacturers can enhance their compliance processes, ensuring that all requisite reports, including Clinical Evaluation Reports (CER) and Post-Market Surveillance Plans (PMS), are readily available and align with regulatory expectations. Understanding these critical guidelines is vital for maintaining compliance and ensuring the safety and efficacy of healthcare products in the European market. Staying informed about changes and updates to these regulations will further empower producers to navigate the complexities of medical device compliance.
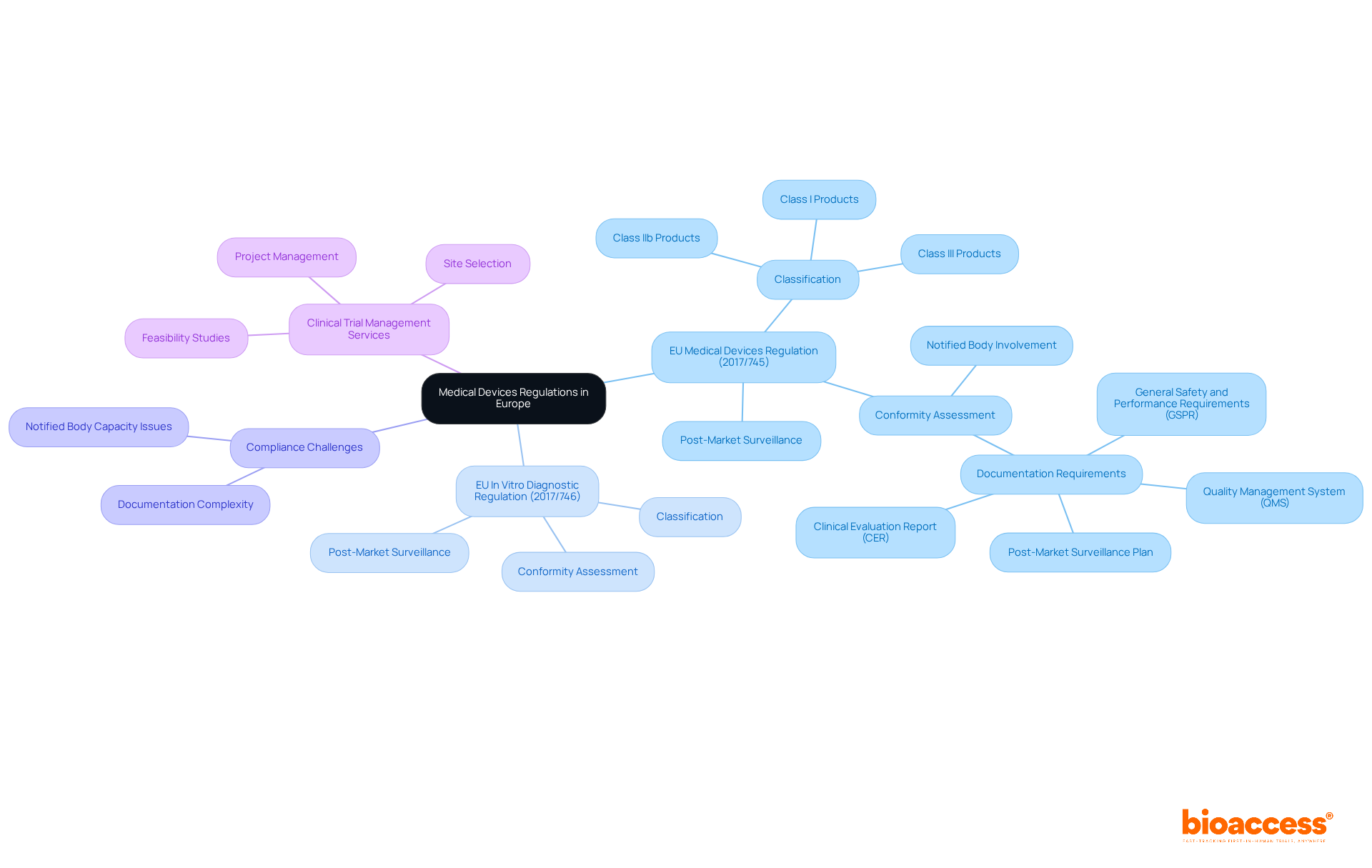
The regulatory procedure for healthcare instruments in the EU, governed by the European Medical Device Regulation, is notably complex, encompassing classification, submission, and approval. Initially, instruments are categorized according to their risk level—Class I, IIa, IIb, or III—thereby establishing the appropriate oversight pathway. A pivotal component of this process is the preparation of a comprehensive Technical Documentation file, which must include detailed design specifications, risk assessments, and clinical data. This documentation is subsequently submitted to a Notified Body for evaluation. Successful submissions culminate in the awarding of a CE mark, facilitating market access within the EU.
It is essential to recognize that all medical devices currently available in the EU must be resubmitted for approval within a timeframe of less than two years, underscoring the urgency of compliance with the European Medical Device Regulation. The significance of Technical Documentation cannot be overstated; it serves as the foundation for approval by authorities and ongoing adherence. In fact, the average time required to secure approval under the new MDR can extend up to 18 months, roughly double the duration needed under previous regulations. This reality accentuates the necessity for meticulous preparation and strict adherence to Technical Documentation requirements.
Moreover, maintaining detailed records and ensuring compliance through post-market monitoring and vigilance reporting is crucial, as oversight organizations may conduct audits and inspections. Companies that excel in their Technical Documentation submissions frequently experience smoother approval processes, highlighting the importance of this critical step in navigating the intricacies of European medical device regulation. Furthermore, nearly 32% of FDA 510(k) submissions did not pass the initial acceptance for review check, revealing the challenges faced in submissions and the importance of careful preparation. Seeking expert regulatory support, such as that provided by Ana Criado, Director of Regulatory Affairs at bioaccess®, is vital for overcoming these complexities and expediting approval, particularly for Medtech, Biopharma, and Radiopharma startups aiming for accelerated clinical trials.
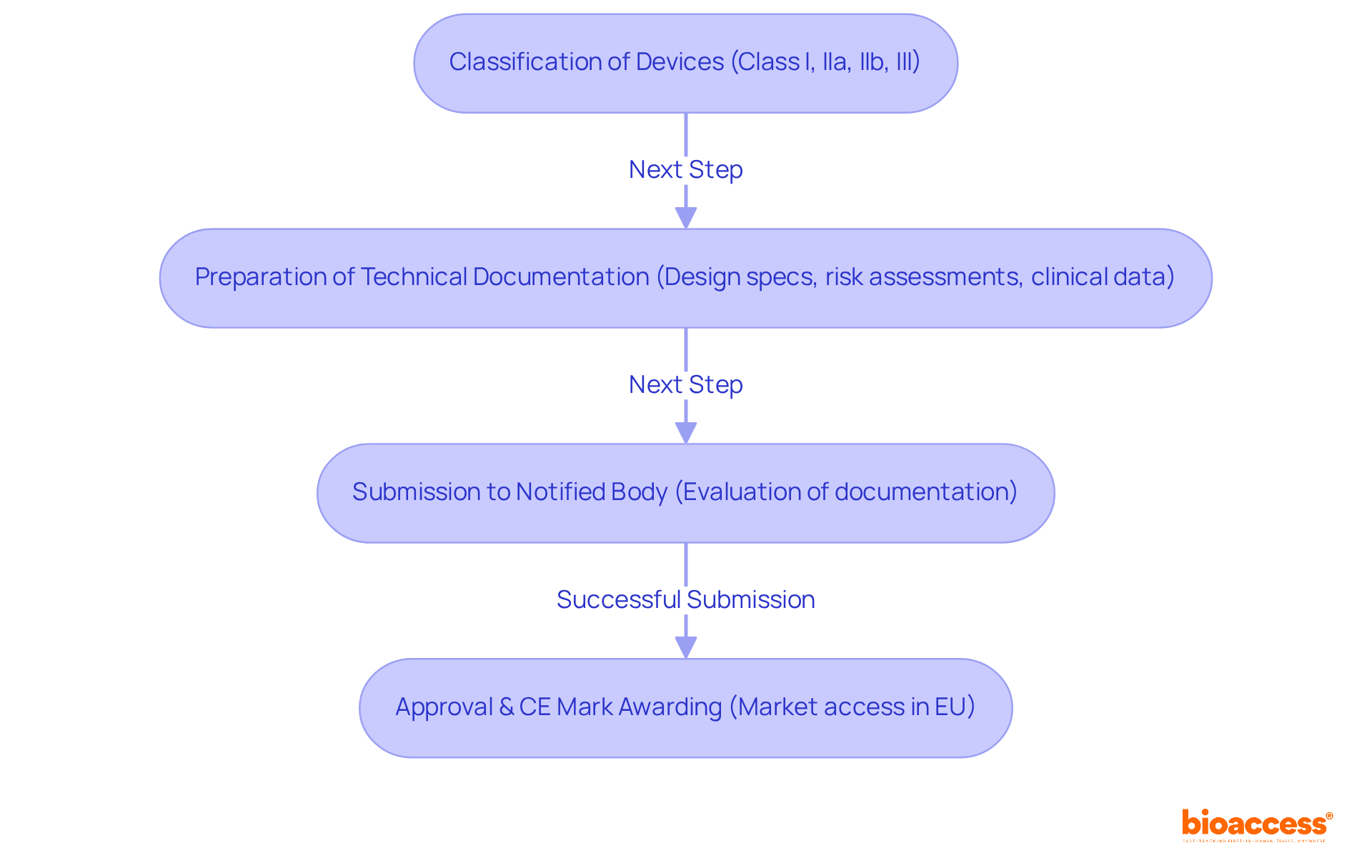
Compliance challenges in clinical research management stem from various factors, such as evolving regulations, inadequate documentation, and communication gaps among stakeholders. It is essential to establish a robust regulatory framework, which includes:
The implementation of technology, particularly Electronic Data Capture (EDC) systems, can significantly enhance adherence rates, reducing error detection time by as much as 75% and data inaccuracies by up to 90% compared to traditional methods. Engaging with regulatory bodies early in the research process offers invaluable insights and guidance, thereby mitigating risks and ensuring studies comply with the European medical device regulation. Furthermore, employing risk-based monitoring (RBM) can lead to a 30% reduction in expenses and a 50% decrease in on-site visits, thereby strengthening regulatory efforts.
Bioaccess offers a comprehensive array of services designed to enhance healthcare equipment trials, which include:
By fostering compliance with the European medical device regulation and leveraging advanced technologies, organizations can effectively navigate the complexities of clinical research, especially in addressing the unique challenges faced by medical device startups.
Understanding and mastering the European Medical Device Regulation (MDR) is essential for achieving clinical success in the healthcare sector. This regulation prioritizes patient safety and product efficacy while establishing a comprehensive framework that manufacturers must navigate to ensure compliance. By grasping the intricacies of the MDR, stakeholders can enhance their strategies and adapt to the evolving landscape, ultimately improving patient outcomes.
Key points highlighted throughout the article include:
The transition from the Medical Devices Directive to the MDR has introduced new challenges and opportunities, particularly concerning classification, submission, and approval processes. Manufacturers must focus on meticulous documentation and the establishment of robust compliance frameworks to navigate these complexities successfully.
The significance of the European Medical Device Regulation extends beyond compliance; it is integral to fostering innovation and ensuring the safety of healthcare products. As the landscape continues to evolve, staying informed about regulatory updates and leveraging advanced technologies will empower manufacturers and researchers alike. Engaging with regulatory bodies early in the process and implementing best practices can mitigate risks and enhance clinical research outcomes, paving the way for successful medical device development in the European market.
What is the European Medical Device Regulation (MDR)?
The European Medical Device Regulation (MDR) establishes a framework that governs the safety and performance of healthcare instruments within the European Union (EU), focusing on patient safety and product efficacy.
What are the key elements of the European Medical Device Regulation?
Key elements include precise definitions of healthcare instruments, clear delineation of stakeholder responsibilities, and the vital role of clinical assessments in the approval process.
Why is compliance with the European Medical Device Regulation important?
Compliance is essential to ensure that healthcare products meet stringent criteria necessary for market access, thereby safeguarding patient safety and product effectiveness.
How does the MDR impact manufacturers of medical devices?
Manufacturers are required to provide comprehensive evidence of compliance through rigorous testing and meticulous documentation to demonstrate adherence to the regulation.
What role do clinical assessments play in the MDR approval process?
Clinical assessments are crucial in the approval process as they evaluate the safety and performance of medical devices before they can be marketed in the EU.
How can understanding the MDR benefit Medtech companies?
A deep understanding of the MDR helps companies ensure compliance, adapt to evolving regulatory requirements, and achieve clinical success in Europe, ultimately enhancing patient outcomes.
What should Medtech innovators focus on to navigate the evolving regulatory landscape?
Medtech innovators should remain informed about patient safety statistics and the implications of the MDR to refine their strategies effectively.