


The article discusses essential steps for mastering the FDA GUDID, underscoring its significance in achieving success in clinical research through effective data management and regulatory compliance. It emphasizes that a thorough understanding of the GUDID's structure, the utilization of its features for data access, and a systematic approach to integration are crucial for enhancing patient safety and optimizing research outcomes. This is evidenced by the outlined processes and best practices for managing device information.
The FDA Global Unique Device Identification Database (GUDID) stands as a foundational pillar in the medical device landscape, ensuring that devices are accurately tracked and identified throughout their lifecycle. For clinical researchers, mastering this database transcends mere compliance; it presents an invaluable opportunity to enhance patient safety and streamline regulatory submissions.
However, the complexities of navigating the GUDID can present significant challenges. How can researchers effectively integrate this essential resource into their practices to maximize its benefits while minimizing errors?
The FDA GUDID stands as a critical asset for the medical device industry, functioning as a centralized repository that guarantees precise identification and tracking of medical devices throughout their lifecycle. By significantly enhancing patient safety, this system plays a pivotal role in mitigating medical errors, optimizing inventory management, and facilitating data-driven decision-making. For clinical researchers, a thorough understanding of the database's structure and purpose is imperative, as it directly influences regulatory submissions and compliance with the FDA GUDID. Familiarity with the system not only fosters smoother interactions with regulatory agencies but also bolsters research outcomes.
Key aspects of the GUDID include:
The implementation of this system has been shown to significantly impact patient safety data, with research indicating that it aids in reducing risks associated with medical equipment errors. As the medical equipment landscape continues to evolve, the FDA GUDID remains a cornerstone for ensuring the safety and effectiveness of products marketed in the U.S.
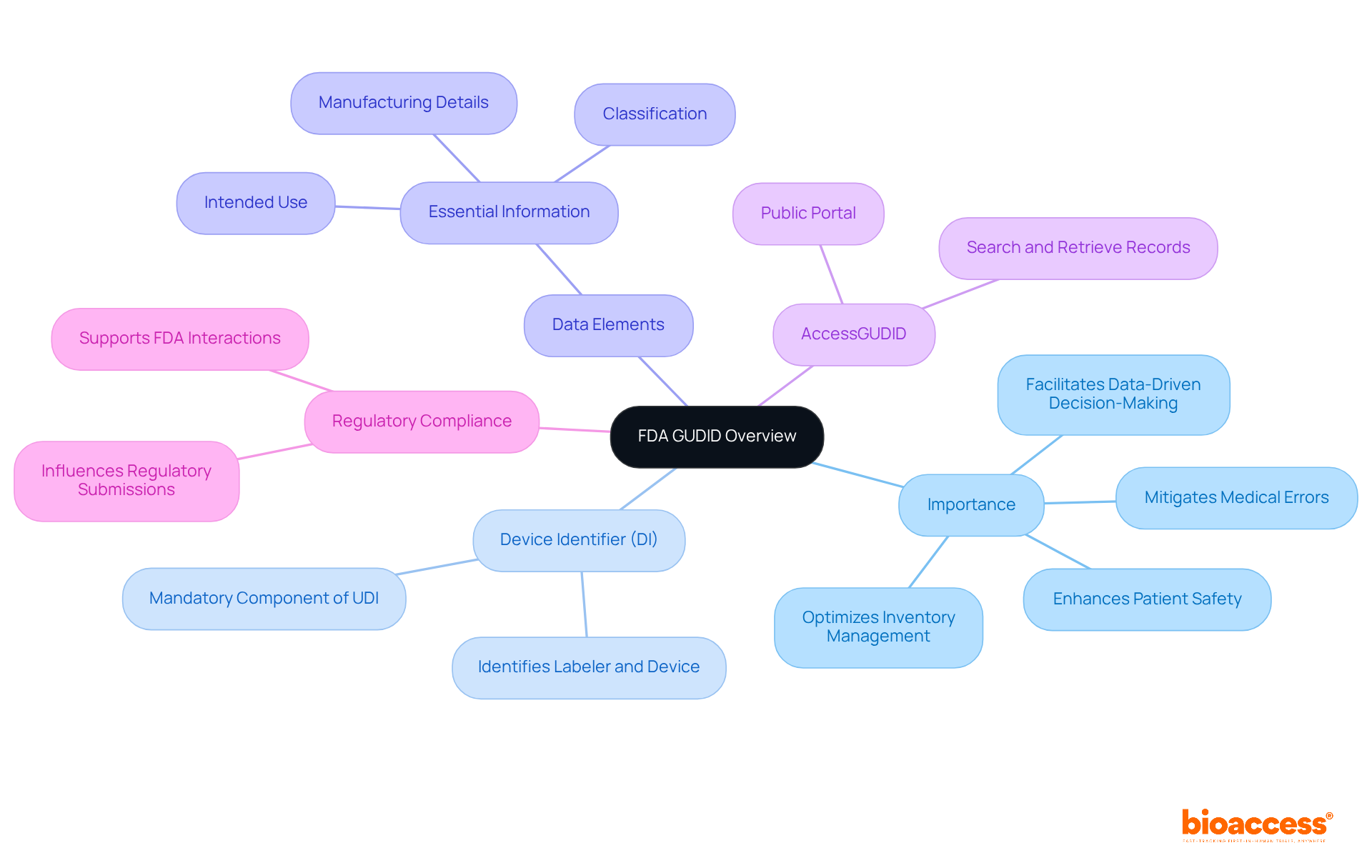
The Global Unique Identification Database (fda gudid) serves as a vital resource for medical product manufacturers and clinical researchers, facilitating data access and management while ensuring compliance and maximizing the utilization of fda gudid data.
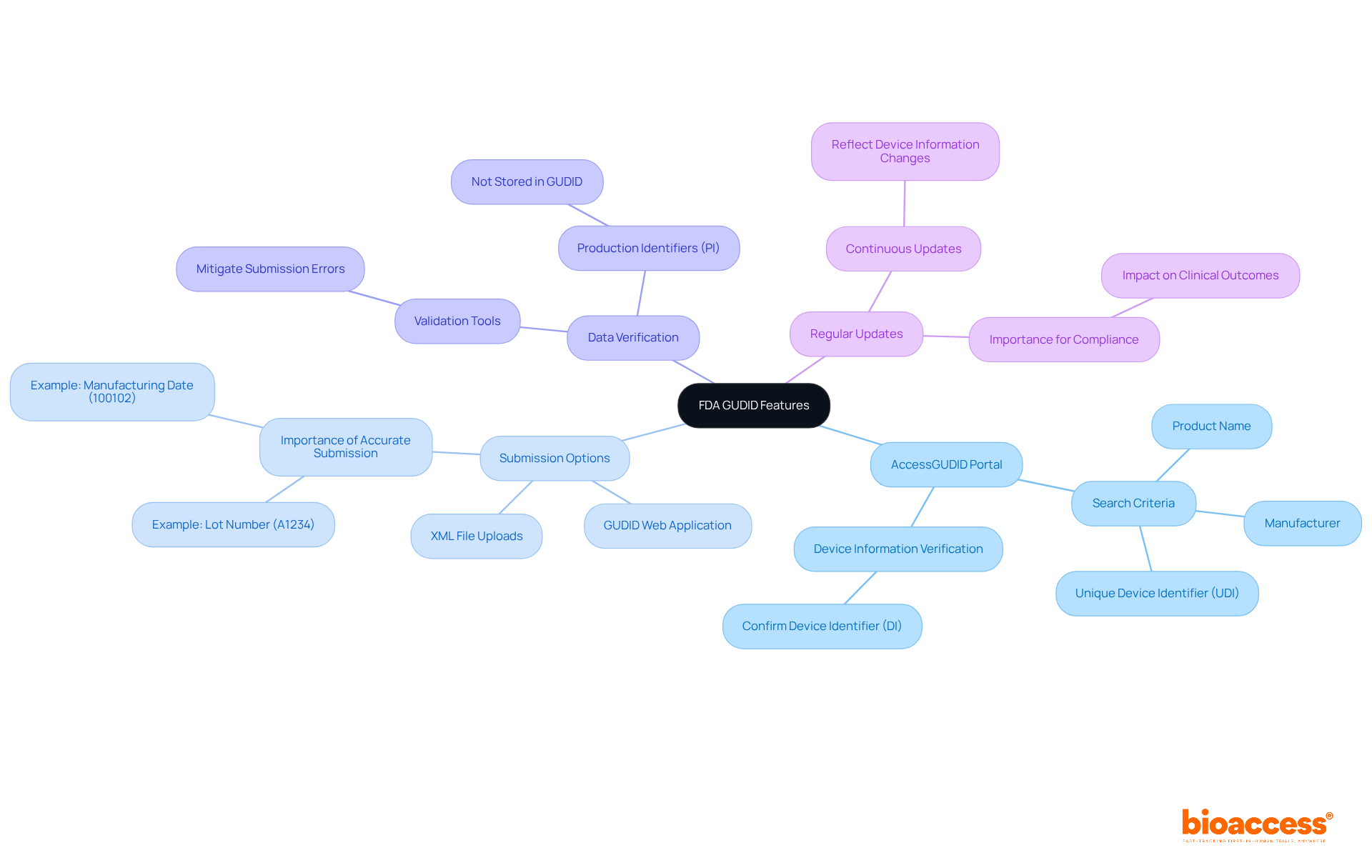
AccessGUDID Portal: This public portal empowers users to search for specific medical products through various criteria, including product name, manufacturer, or Unique Device Identifier (UDI). Researchers can utilize this tool to verify device information, ensuring that their submissions align with existing records. For instance, a manufacturer can quickly confirm the Device Identifier (DI) associated with their product, facilitating accurate information entry. The DI is the primary key for retrieving information in the database, making it essential for effective information management.
Submission Options: Manufacturers enjoy the flexibility of providing their GUDID information via the GUDID Web Application or through XML file uploads. Familiarity with these submission methods is crucial for optimizing the process and ensuring precise information entry, which is imperative for regulatory compliance. For example, a producer may submit a Lot Number (A1234) and a Manufacturing Date (100102) alongside their Device Identifier (DI) (12345678901234) to ensure comprehensive information representation.
Data Verification: The FDA offers validation tools that ensure submitted information conforms to the fda gudid established frameworks. Researchers should leverage these tools to mitigate common submission errors, thereby upholding data integrity and enhancing the reliability of their submissions. It is noteworthy that while Production Identifiers (PI) are numeric or alphanumeric codes identifying production information for a product, they are neither submitted to nor stored in the Global Unique Device Identification Database.
Regular Updates: The database undergoes continuous updates to reflect alterations in device information, such as recalls or modifications. Staying informed about these updates is essential for maintaining regulatory compliance and ensuring patient safety, as timely access to accurate information can significantly impact clinical outcomes. The Pew Charitable Trusts has highlighted the importance of integrating relevant information with current FDA databases, which can enhance resource utilization and information accessibility, further underscoring the necessity of remaining updated on changes.
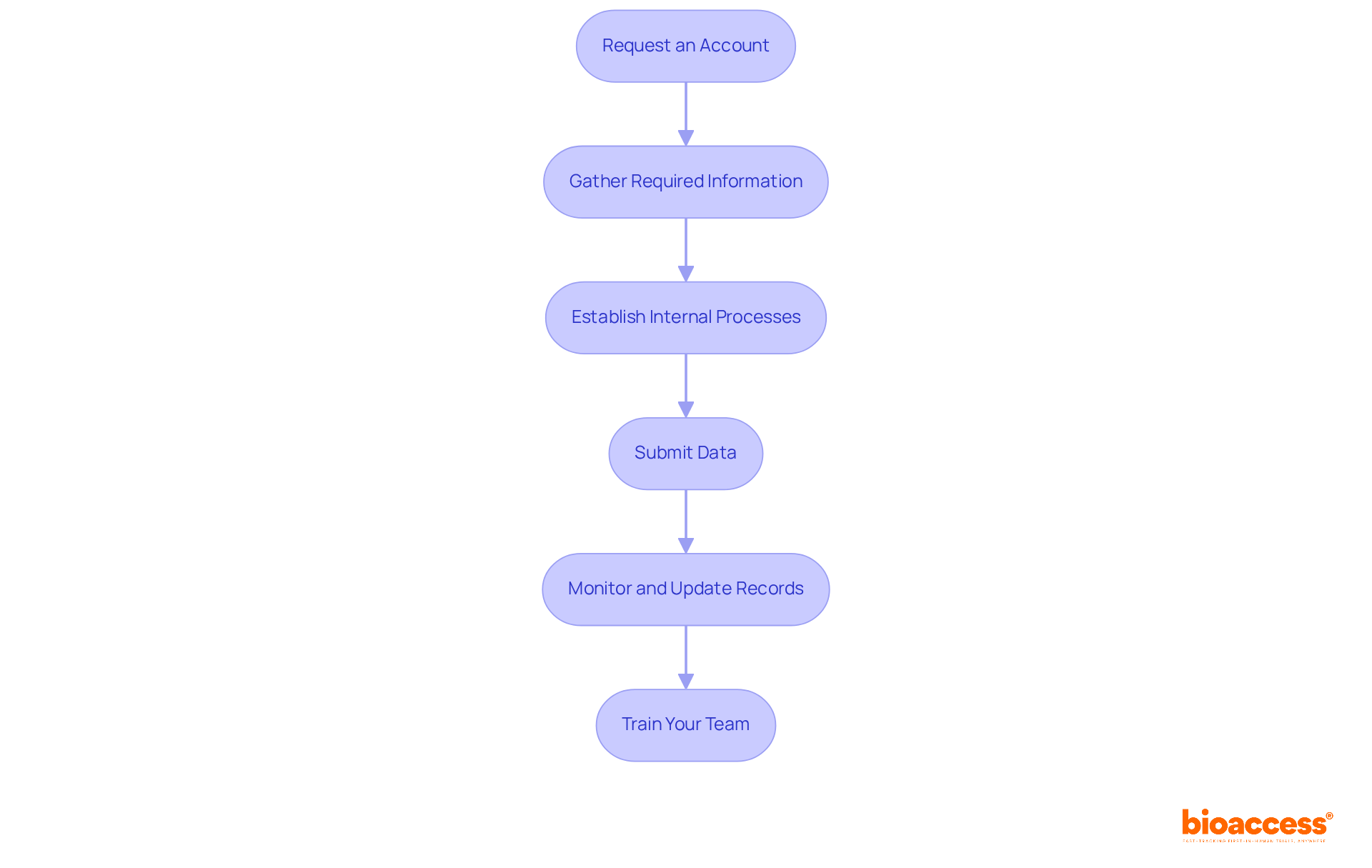
Incorporating unique device identification information into clinical research processes requires a systematic approach to ensure compliance and enhance information management practices. The following essential steps must be adhered to:
Request an Account: Initiate the process by requesting an account from the FDA GUDID, which is essential for submitting product information and accessing related data.
Gather Required Information: Compile all necessary information elements for your devices, including the Device Identifier (DI), device description, intended use, and manufacturer details. Accuracy and completeness are paramount at this stage.
Establish Internal Processes: Develop standard operating procedures (SOPs) for managing the required information. Clearly define roles and responsibilities within your team for information entry, validation, and submission to optimize operations.
Submit Data: Use the web application or XML file upload for submitting product information. Ensure that all information complies with the schema to prevent submission errors, as minor discrepancies can lead to significant regulatory challenges.
Monitor and Update Records: Regularly review and update your device records to reflect any changes in device information. This practice is critical for maintaining compliance and ensuring that your data remains current.
Train Your Team: Conduct training sessions for your team on FDA GUDID requirements and best practices for information management. This will foster a culture of accuracy and compliance, ensuring that all team members appreciate the importance of precise information submission.
Successful examples from clinical research organizations demonstrate that adhering to these steps not only facilitates compliance but also enhances overall data accuracy. Statistics reveal that maintaining consistent information across databases significantly mitigates the risk of discrepancies, which can otherwise result in compliance failures. By following this structured approach, organizations can effectively navigate the complexities of GUDID integration and contribute to the integrity of the medical device industry.
Mastering the FDA GUDID is essential for ensuring the success of clinical research in the medical device industry. This centralized database not only enhances patient safety by enabling precise identification and tracking of devices but also plays a crucial role in regulatory compliance and data management. A comprehensive understanding of the GUDID is vital for researchers to navigate the complexities of medical device regulations effectively.
The article delves into the key features of the FDA GUDID, including:
It emphasizes the steps required for successful integration of GUDID data into clinical research processes, such as:
By following these structured approaches, researchers can significantly mitigate compliance risks and enhance the accuracy of their submissions.
Ultimately, the FDA GUDID stands as a cornerstone in the medical device landscape, reinforcing the importance of data integrity and regulatory adherence. As the industry evolves, staying informed about GUDID updates and best practices will be essential for researchers aiming to contribute to patient safety and effective medical device utilization. Engaging with the GUDID not only supports compliance but also empowers researchers to make informed, data-driven decisions that can lead to improved clinical outcomes.
What is the FDA GUDID?
The FDA GUDID is a centralized repository for the medical device industry that ensures precise identification and tracking of medical devices throughout their lifecycle.
How does the FDA GUDID enhance patient safety?
The FDA GUDID enhances patient safety by mitigating medical errors, optimizing inventory management, and facilitating data-driven decision-making.
Why is it important for clinical researchers to understand the FDA GUDID?
A thorough understanding of the FDA GUDID is imperative for clinical researchers as it influences regulatory submissions and compliance, leading to smoother interactions with regulatory agencies and improved research outcomes.
What is the Device Identifier (DI) in the context of the FDA GUDID?
The Device Identifier (DI) is a mandatory, fixed component of the Unique Device Identifier (UDI) that identifies both the labeler and the specific medical device.
What kind of information is included in the data elements of the FDA GUDID?
Each unit's record in the FDA GUDID includes essential information such as intended use, classification, and manufacturing details.
What is AccessGUDID?
AccessGUDID is a public portal that allows users to search for specific medical devices and retrieve their associated records.
What impact does the FDA GUDID have on patient safety data?
The implementation of the FDA GUDID has been shown to significantly impact patient safety data by reducing risks associated with medical equipment errors.
Why is the FDA GUDID considered a cornerstone for medical products in the U.S.?
The FDA GUDID is considered a cornerstone for ensuring the safety and effectiveness of medical products marketed in the U.S. as the medical equipment landscape continues to evolve.