


The article emphasizes critical elements of Health Canada medical device regulation that Medtech companies must master for successful market entry. It underscores the necessity of understanding device classifications, adhering to regulatory requirements, and engaging with Health Canada throughout the product development process.
These elements collectively enhance compliance and accelerate market readiness, making them indispensable for any Medtech firm aiming to navigate the complexities of the regulatory landscape.
Navigating the complex landscape of medical device regulation in Canada is essential for Medtech companies striving for success. With Health Canada leading the charge, the regulatory framework classifies devices by risk, determining the level of scrutiny and evidence necessary for market approval. Recent changes, such as the introduction of the Medical Device Single Audit Program, are reshaping compliance processes, presenting companies with the urgent challenge of adapting to ensure their products not only meet safety and efficacy standards but also align with the evolving regulations.
How can Medtech firms effectively master these regulations to secure their foothold in a competitive market?
In Canada, the oversight of medical equipment is governed by the Health Canada medical device regulation, alongside the Food and Drugs Act and the Medical Devices Regulations (MDR). The MDR categorizes items into four groups according to risk:
Each classification involves specific requirements related to safety, effectiveness, and quality. This understanding is essential for Medtech companies, as it determines the degree of regulatory oversight and the kind of evidence needed for approval. For instance, higher-risk products necessitate a Medical Product License (MDL), which entails a thorough examination of clinical data and quality management systems. In contrast, lower-risk products may only require a more straightforward notification process. Such comprehension of the Health Canada medical device regulation is crucial for effectively navigating the regulatory environment, ensuring compliance, and facilitating successful market entry.
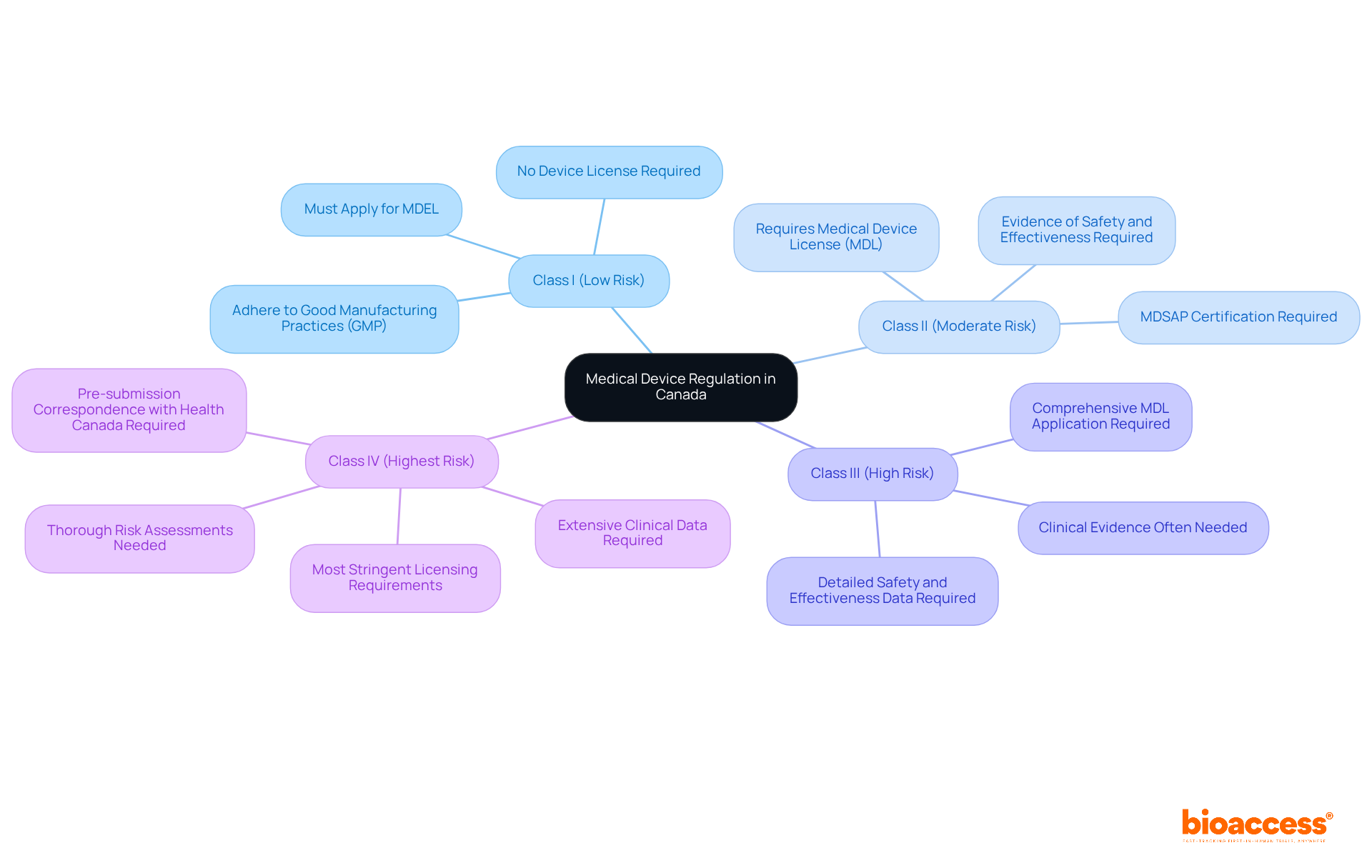
The federal agency serves as the authoritative body overseeing the regulation of medical instruments within the nation, placing critical emphasis on the assessment of safety, efficacy, and quality prior to market introduction. This department meticulously reviews pre-market submissions and conducts ongoing post-market surveillance to ensure compliance with the Health Canada medical device regulation. Medical instruments are classified into four distinct categories based on risk level:
As of 2025, compliance rates for medical products reflect significant progress, underscoring the effectiveness of the Health Canada medical device regulation established by the health authority. In instances of severe non-compliance, the department possesses the authority to suspend or revoke a product license, highlighting the gravity of regulatory adherence. Furthermore, the national health agency offers guidance documents that delineate expectations for manufacturers, which are essential for Medtech firms in preparing their submissions. Engaging with the federal health agency during the early stages of product development not only streamlines navigation through the Health Canada medical device regulation but also provides companies with insights into the specific requirements tied to their product's classification and intended use. Recent initiatives, including enhanced monitoring and the mandatory reporting of incidents—where hospitals are required to report a medical device incident within 30 days—underscore the commitment of authorities to uphold high safety standards while fostering innovation within the Medtech sector.
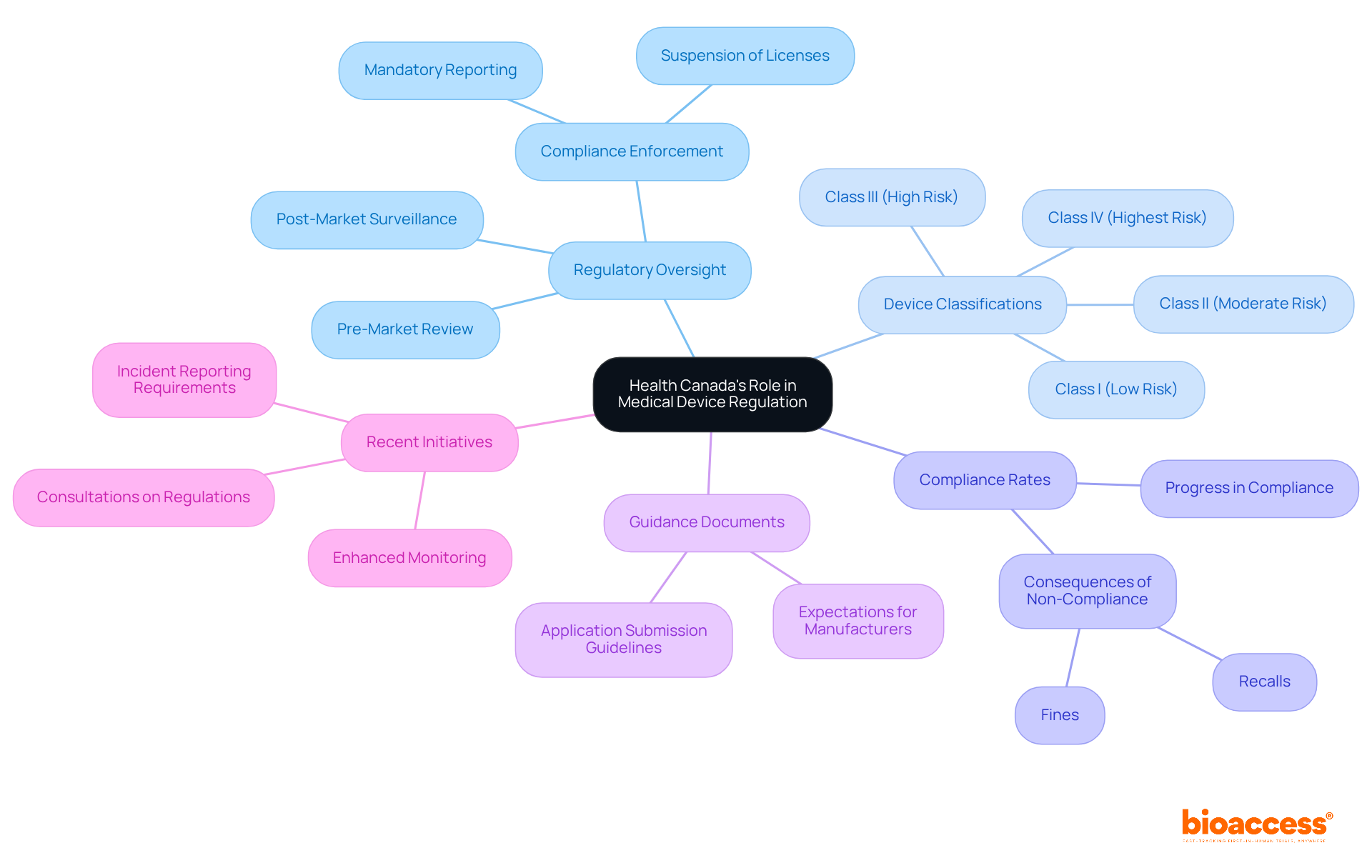
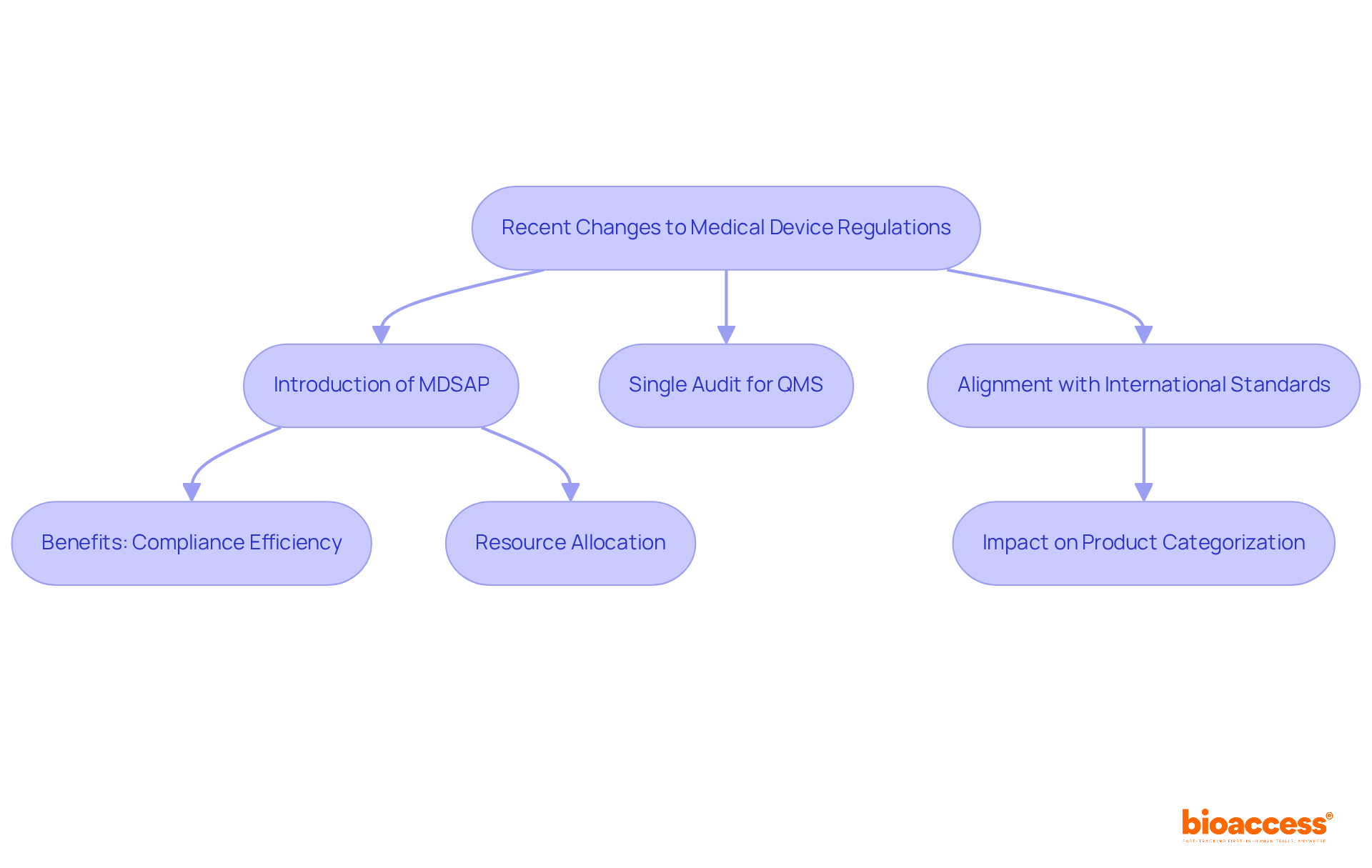
Recent updates to the Medical Device Regulations have introduced the Medical Device Single Audit Program (MDSAP), which empowers manufacturers to undergo a single audit that satisfies the quality management system (QMS) requirements of various regulatory authorities, including the Health Canada medical device regulation, the FDA, and others. This streamlined approach significantly enhances compliance efficiency, allowing manufacturers to allocate resources more effectively and reduce time to market.
As James Stoia observes, "MDSAP is acknowledged by authorities including the U.S. Food and Drug Administration (FDA), the Canadian authorities (HC), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), Brazil’s ANVISA, and the Australian Therapeutic Goods Administration (TGA)."
Moreover, the Health Canada medical device regulation has been updated to better align with international standards, impacting how products are categorized and the evidence required for their approval. Companies adapting to MDSAP have reported improved operational efficiencies and product quality, demonstrating the program's positive influence on Medtech compliance.
Additionally, the transition period for the Medical Device Regulation (MDR) has been extended until 2027/2028, making it crucial for Medtech companies to stay informed about these regulatory changes to ensure their products meet the latest requirements and mitigate potential delays in market access.
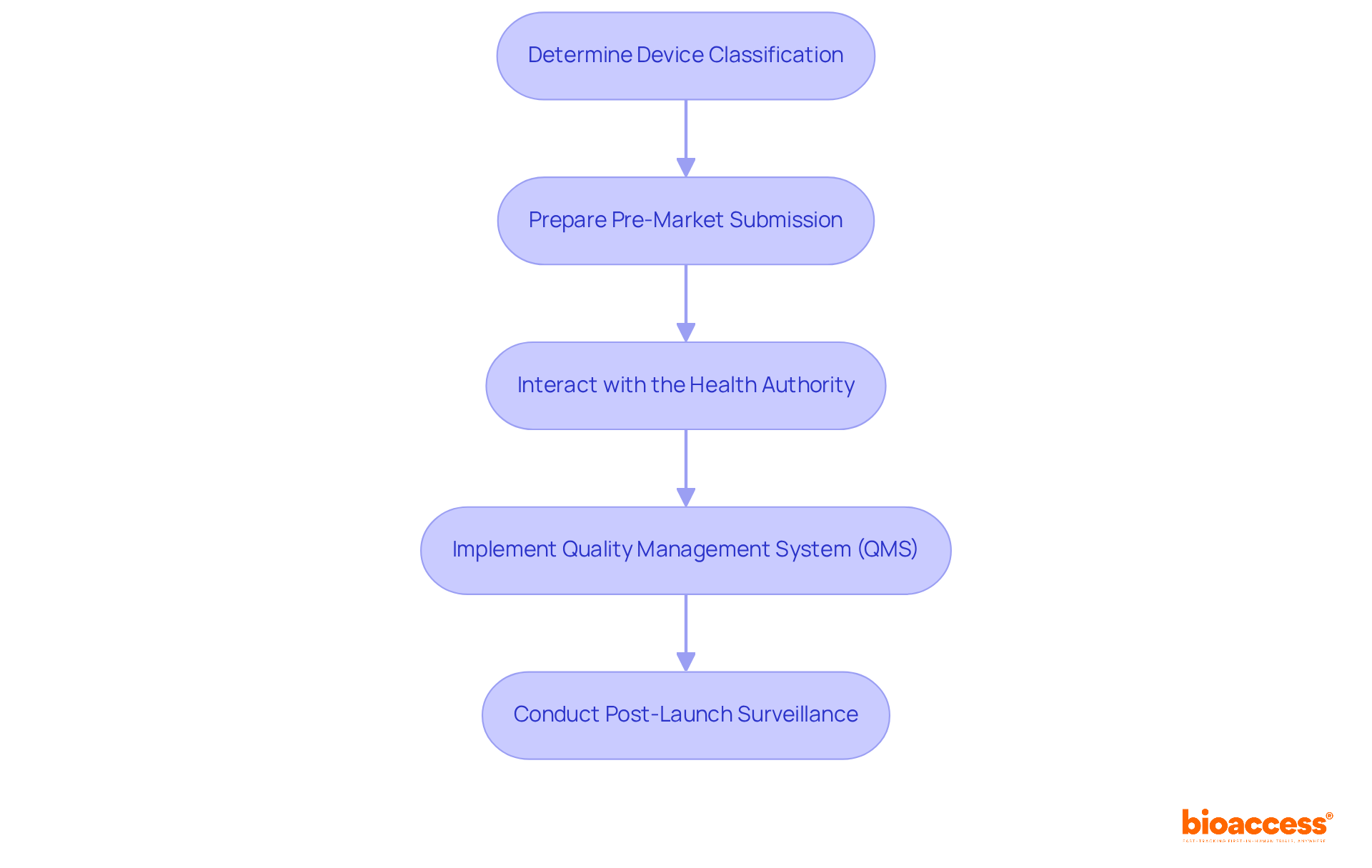
To effectively navigate compliance with Canadian medical device regulations, Medtech companies should adhere to the following steps:
Determine Device Classification: Assess the device's risk level to identify the appropriate classification and regulatory pathway. This classification is essential, as it determines the regulatory requirements and the pace of entry into the industry. Statistics suggest that accurate classification can decrease time to release by as much as 30%.
Prepare Pre-Market Submission: Compile comprehensive documentation, including safety and efficacy data. A Medical Equipment License (MDL) application is essential for products classified as Class II, III, or IV under the health canada medical device regulation, ensuring that only items that meet stringent safety and effectiveness standards reach the market. For instance, the case study titled "Canadian Medical Device License: A Prerequisite" highlights that obtaining an MDL is essential before applying for an MDEL, ensuring thorough evaluation of safety and effectiveness.
Interact with the Health Authority: Consider pre-submission discussions with the regulatory body to clarify requirements and expectations. This proactive approach can significantly streamline the submission process and enhance the likelihood of approval. As noted in industry feedback, many manufacturers found that early engagement with regulatory bodies improved their submission outcomes.
Implement Quality Management System (QMS): Establish a QMS that complies with ISO 13485 standards. This system is crucial for upholding product quality and ensuring continuous regulatory compliance throughout the item's lifecycle. Companies with robust QMS practices report a 40% higher success rate in regulatory approvals.
Conduct Post-Launch Surveillance: Following entry into the marketplace, continuously observe product performance and report any negative incidents to Health Canada. This vigilance not only guarantees adherence but also enhances the overall safety and effectiveness of medical devices available. The significance of post-commercial monitoring is highlighted by the quote, 'Medical evidence is the currency in access,' stressing the necessity for continuous data gathering and evaluation.
By following these steps, Medtech companies can significantly enhance their chances of successful compliance with the health canada medical device regulation and market entry in Canada. Successful pre-market submissions by companies that have engaged in thorough classification assessments and maintained robust quality management practices demonstrate the effectiveness of this approach.
Understanding and mastering the Health Canada medical device regulation is essential for Medtech companies striving for success in the competitive landscape of medical technology. By grasping the nuances of device classification, regulatory pathways, and compliance requirements, businesses can effectively navigate the complexities of the regulatory environment. This ensures that their products not only meet safety and efficacy standards but also achieve timely market access.
Key insights highlighted throughout the article include:
Furthermore, the introduction of the Medical Device Single Audit Program (MDSAP) and recent regulatory updates serve to streamline compliance and align with international standards, thereby enhancing operational efficiencies for manufacturers.
In conclusion, the significance of adhering to the Health Canada medical device regulation cannot be overstated. Medtech companies are strongly encouraged to adopt a proactive approach, remaining informed about regulatory changes and implementing robust quality management systems. By prioritizing compliance and engaging with regulatory authorities, these companies can ensure not only the safety and effectiveness of their products but also foster innovation that ultimately benefits patients and healthcare providers alike.
What governs the regulation of medical devices in Canada?
The regulation of medical devices in Canada is governed by Health Canada medical device regulation, the Food and Drugs Act, and the Medical Devices Regulations (MDR).
How are medical devices categorized in Canada?
Medical devices in Canada are categorized into four groups according to risk: Class I (low risk), Class II (moderate risk), Class III (high risk), and Class IV (highest risk).
What is the significance of the classification of medical devices?
The classification of medical devices determines the degree of regulatory oversight and the type of evidence required for approval, which is essential for Medtech companies to understand.
What are the requirements for higher-risk medical devices?
Higher-risk medical devices require a Medical Product License (MDL), which involves a thorough examination of clinical data and quality management systems.
What is required for lower-risk medical devices?
Lower-risk medical devices may only require a more straightforward notification process instead of extensive approval requirements.
Why is understanding Health Canada medical device regulation important?
Understanding Health Canada medical device regulation is crucial for navigating the regulatory environment, ensuring compliance, and facilitating successful market entry for medical devices.