


The article emphasizes the essential steps for mastering Informed Consent Forms (ICFs) in clinical trials, highlighting their critical role in ensuring ethical research practices and participant understanding. It underscores the necessity of clear communication and details the critical elements of ICFs, alongside the structured process of obtaining consent. Effective ICFs not only foster trust but also protect participants' rights, which is vital for the success of clinical trials. This understanding is paramount as it reinforces the ethical framework within which clinical research operates.
Informed Consent Forms (ICFs) are fundamental to the ethical framework of clinical trials, ensuring that participants possess a comprehensive understanding of their involvement. As clinical research continues to evolve, the importance of these documents is increasingly recognized, particularly as regulatory bodies advocate for enhanced clarity and participant engagement.
Despite this critical role, an astonishing 95% of eligible patients remain disengaged from clinical trials. This statistic prompts a pivotal question: how can researchers leverage ICFs to improve communication and build trust, ultimately bridging the gap in participation?
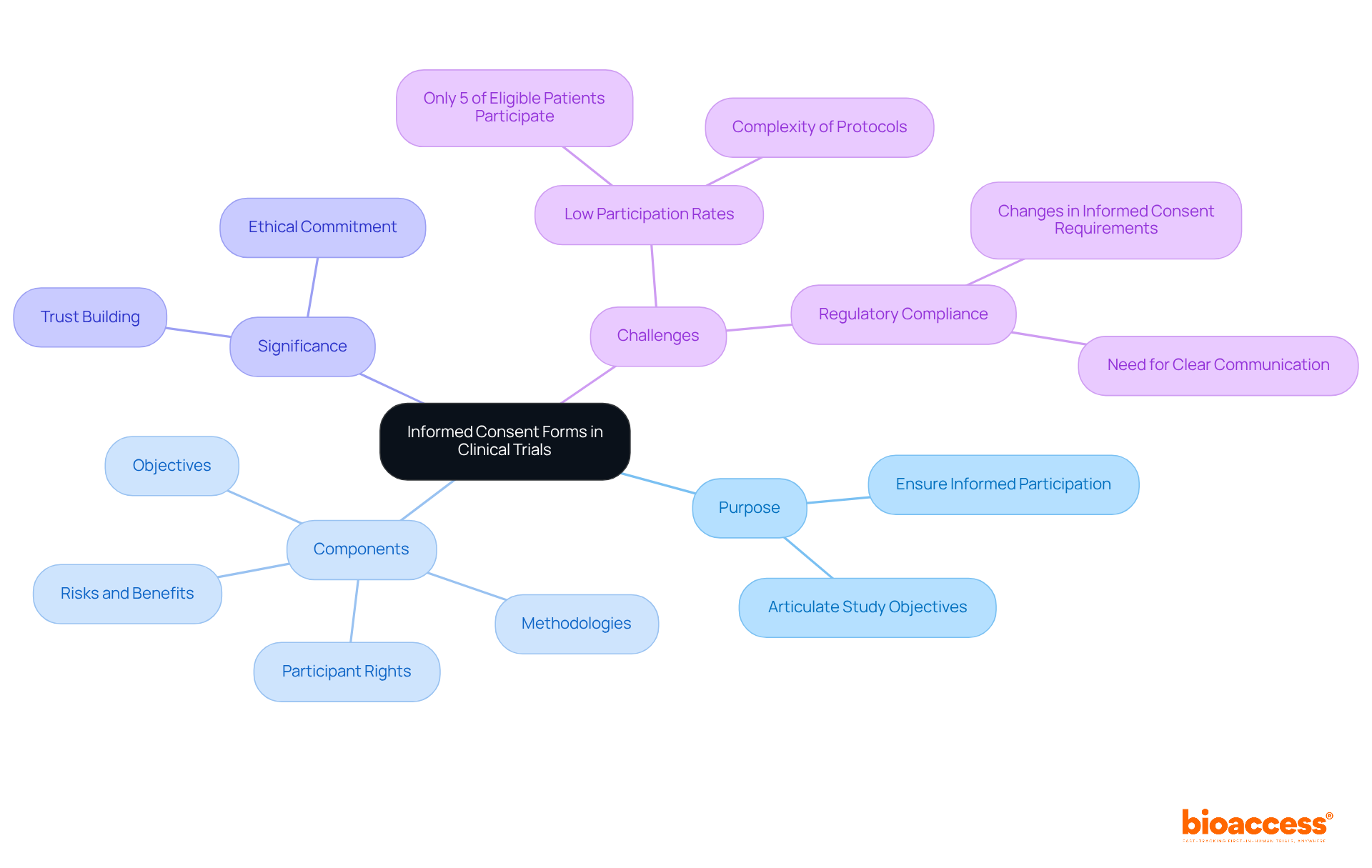
Consent Forms (ICFs) are essential documents in clinical trials, ensuring that individuals are fully informed about the research they are considering. These forms articulate the study's objectives, the methodologies employed, potential risks and benefits, and the rights of the participants. ICFs are not merely a regulatory requirement; they represent a fundamental ethical commitment, empowering individuals to make informed choices about their involvement in research.
The significance of ICFs extends beyond mere compliance; they are pivotal in fostering trust between researchers and participants. As we move into 2025, the emphasis on clear and comprehensible consent materials becomes increasingly critical, with regulatory bodies striving to enhance participant understanding and engagement. For example, the recent mandate for a concise summary of key information at the beginning of consent forms is designed to improve clarity, in line with the FDA's proposed guidance on informed consent practices.
Statistics reveal that a substantial proportion of clinical trials utilize ICFs, underscoring their vital role in the research process. However, it is important to note that only 5% of eligible patients participate in clinical trials, a statistic that has remained consistent for three decades. This highlights the urgent need for effective communication through ICFs, as the complexity of clinical trial protocols often complicates the identification of suitable candidates. As the landscape of clinical studies continues to evolve, the importance of ICFs in safeguarding rights and upholding ethical standards remains a cornerstone of successful clinical trials.
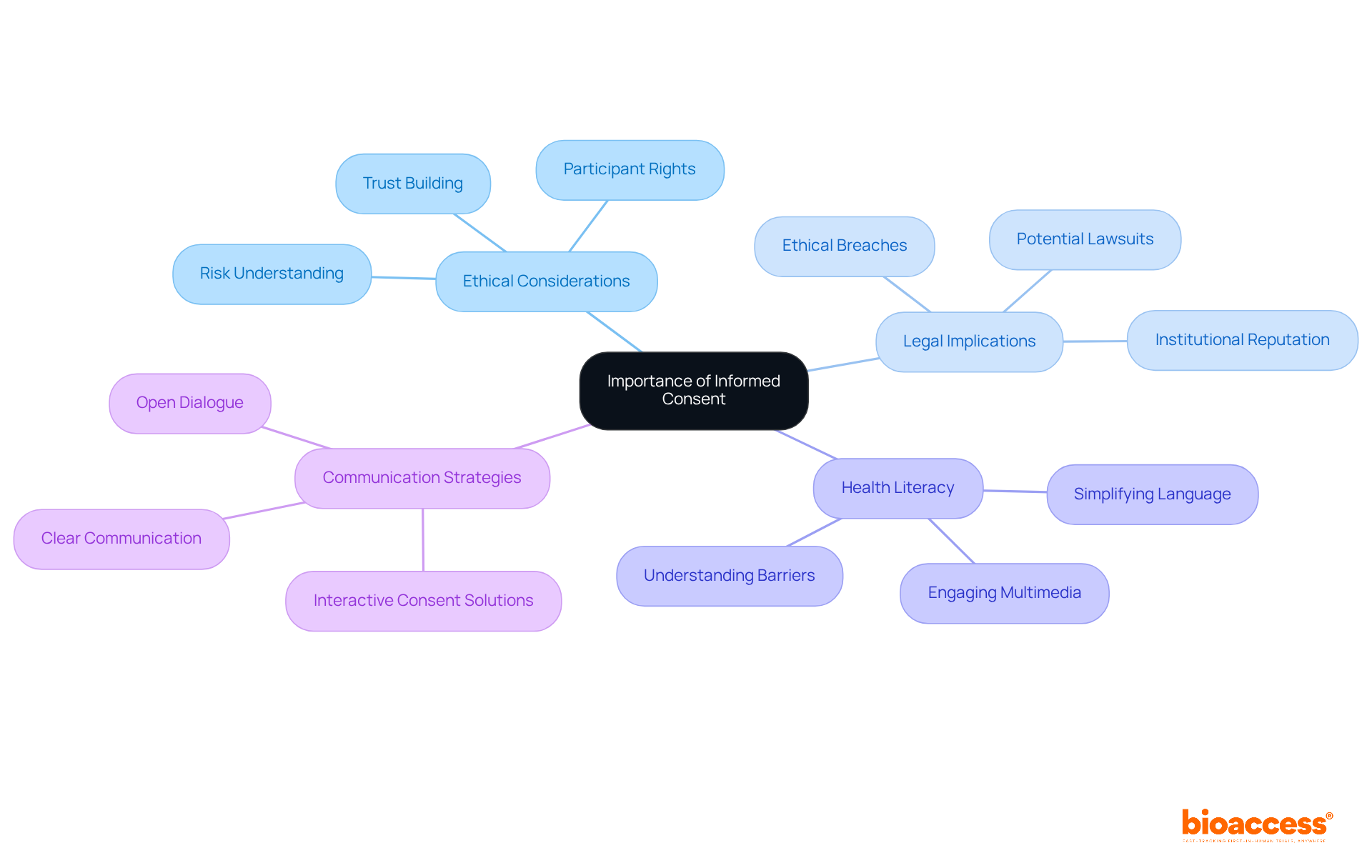
Informed agreement is crucial to ethical research in an icfs clinical trial, serving as a safeguard for individuals by ensuring they comprehend the study's nature, including associated risks and benefits. This comprehension empowers individuals to make voluntary and informed decisions regarding their participation. The approval process not only protects individuals' rights but also cultivates trust between researchers and subjects, which is essential for achieving successful outcomes in the icfs clinical trial.
Legally, obtaining informed agreement is imperative in an icfs clinical trial; failure to do so can result in significant ethical breaches and legal ramifications, including potential lawsuits and damage to institutional reputations. For instance, a systematic review revealed that fewer than 10% (n=34; 5.3%) of studies adequately reported evaluations of individuals' capacity to provide consent, highlighting a concerning gap in ethical compliance.
Furthermore, a substantial portion of the U.S. adult population struggles with health literacy, creating barriers to understanding voluntary consent. In an icfs clinical trial, the lack of informed agreement can erode participant trust, as individuals may feel misled or exploited, particularly in vulnerable populations.
Informed agreement is vital for protecting those interested in participating in studies. Consequently, researchers must prioritize clear communication and ethical practices, including leveraging advancements in technology such as electronic consent solutions that enhance the informed consent process in icfs clinical trial by offering interactive multimedia experiences, thereby preserving the integrity of clinical trials.
An effective Informed Consent Form (ICF) must encompass several essential elements to ensure clarity and compliance with ethical standards in clinical research:
By incorporating these elements, researchers can create agreements that not only meet regulatory requirements but also improve understanding and involvement in the study process.

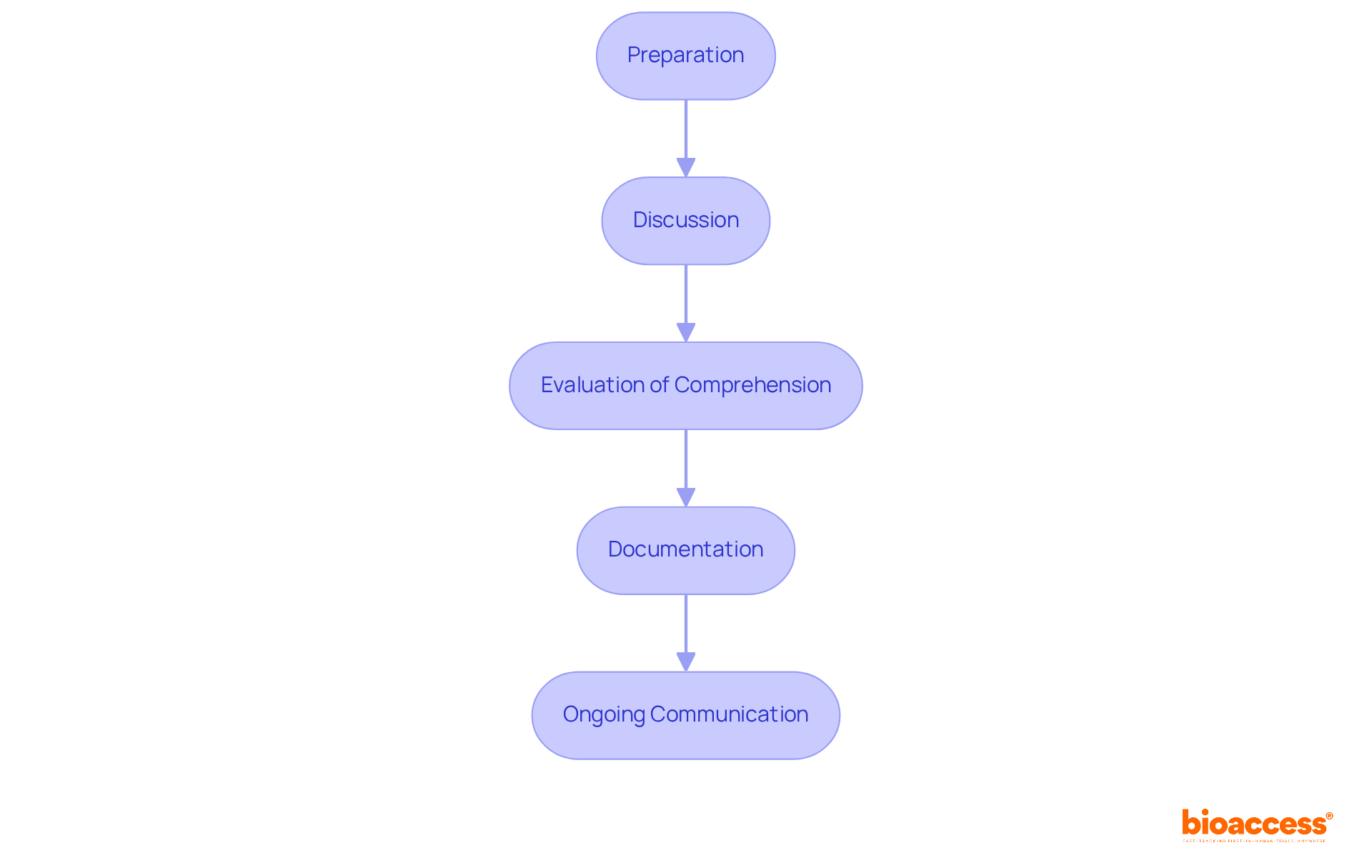
The process of obtaining informed consent is essential for ethical clinical research and involves several key steps:
Preparation: Before interacting with potential subjects, ensure a thorough understanding of the study and the Informed Consent Form (ICF). This foundational knowledge is crucial for effective communication.
Discussion: Conduct a detailed conversation with the individual about the study, utilizing the ICF as a reference. It is essential to convey information in clear, accessible language, as studies suggest that most individuals find the ICF easy to grasp, while some research staff raise concerns about understanding. The mean time taken for the last informed consent discussion was 51 minutes, highlighting the time commitment involved.
Evaluation of Comprehension: Verify attendees' understanding by posing open-ended inquiries. This step is essential, as sufficient time for consideration can vary from hours to days, enabling individuals to contemplate the information given. It is also important to allocate sufficient time for informed agreement discussions and consider using Teach Back/Talk Back techniques to enhance understanding.
Documentation: Once the individual agrees to take part, record their approval by having them sign and date the ICF. An individual is not deemed registered until both they and the researcher have signed the agreement form. Furthermore, a witness signature is necessary when acquiring approval from individuals who are physically unable to read, write, speak, or are visually impaired.
Ongoing Communication: Maintain open lines of communication throughout the study. Participants should feel motivated to ask questions and express concerns at any time, reinforcing their understanding and comfort with the study process.
By adhering to these steps, researchers can improve the awareness process, ensuring that participants are knowledgeable and their rights are safeguarded throughout the study. Furthermore, 75% of research staff reported that a shorter and simpler document would improve the informed consent process, emphasizing the need for clarity and conciseness in the ICF.
Informed Consent Forms (ICFs) are essential in the ethical landscape of clinical trials, functioning both as a regulatory requirement and a tool for empowering participants. By ensuring individuals are fully informed about the study's objectives, methodologies, risks, and their rights, ICFs cultivate a transparent relationship between researchers and participants. As the clinical research environment advances, the demand for clear and comprehensible consent materials becomes increasingly critical.
This article underscores several pivotal aspects of ICFs, particularly their role in safeguarding participant rights and fostering trust in the research process. Key elements of an ICF, including:
are vital for enhancing participant understanding and compliance. Moreover, the outlined process for obtaining informed consent emphasizes the necessity of effective communication, comprehension evaluation, and continuous dialogue, all of which are essential for upholding ethical standards throughout the study.
Ultimately, prioritizing informed consent transcends mere regulatory compliance; it embodies respect for individuals' autonomy and the cultivation of trust within clinical research. As researchers seek to enhance participant engagement and understanding, it is imperative to adopt best practices that guarantee clarity and transparency in ICFs. Embracing these principles will not only fortify the integrity of clinical trials but also contribute significantly to the advancement of medical knowledge and patient care.
What are Informed Consent Forms (ICFs) in clinical trials?
Informed Consent Forms (ICFs) are essential documents that ensure individuals are fully informed about the research they are considering. They detail the study's objectives, methodologies, potential risks and benefits, and the rights of the participants.
Why are ICFs important in clinical trials?
ICFs are crucial not only for regulatory compliance but also as an ethical commitment that empowers individuals to make informed decisions about their involvement in research. They foster trust between researchers and participants.
What recent changes have been made to ICFs to improve participant understanding?
Recent mandates require a concise summary of key information at the beginning of consent forms to enhance clarity, in line with the FDA's proposed guidance on informed consent practices.
What statistics highlight the role of ICFs in clinical trials?
A significant proportion of clinical trials utilize ICFs; however, only 5% of eligible patients participate in clinical trials, a statistic that has remained consistent for three decades. This emphasizes the need for effective communication through ICFs.
How do ICFs contribute to participant engagement in clinical trials?
By providing clear and comprehensible information, ICFs help improve participant understanding and engagement, which is increasingly critical as the landscape of clinical studies evolves.
What challenges do ICFs address in the context of clinical trials?
ICFs address the complexity of clinical trial protocols, which often complicates the identification of suitable candidates. They play a vital role in safeguarding participants' rights and upholding ethical standards in research.