


This article examines the comprehensive framework of medical device regulations in Mexico, which is governed by the General Health Law and enforced by COFEPRIS. The regulations categorize devices into three distinct risk classifications. Understanding these regulations is paramount, as it encompasses the approval process and post-registration obligations. Such knowledge is crucial for manufacturers seeking to successfully enter and navigate the rapidly growing Mexican healthcare market.
Navigating the intricate landscape of medical device regulations in Mexico presents a significant challenge for manufacturers and stakeholders. With a rapidly expanding market projected to reach $5.5 billion, grasping the legal framework governed by COFEPRIS is essential for achieving success. This comprehensive guide explores the complexities of medical device registration, providing insights into:
How can manufacturers ensure they meet these evolving standards while seizing market opportunities?
The medical device regulations in Mexico are primarily governed by the General Health Law (Ley General de Salud) and enforced by the Federal Commission for the Protection against Sanitary Risk (COFEPRIS). These regulations categorize medical instruments into three risk classifications:
This classification is critical as it impacts the entry process, documentation requirements, and timelines for market access. Starting in 2025, the Acuerdo has set forth new mandates for low-risk products, necessitating sanitary registration and compliance with Good Manufacturing Practices (GMP).
With nearly 90% of healthcare instruments sold in Mexico being imports, producers must remain vigilant regarding medical device regulations in Mexico to ensure compliance and prevent delays in accessing this rapidly expanding market, which is projected to reach US$5.5 billion. Staying informed about regulatory updates from the health authority is essential for effectively navigating the Mexican healthcare product landscape.
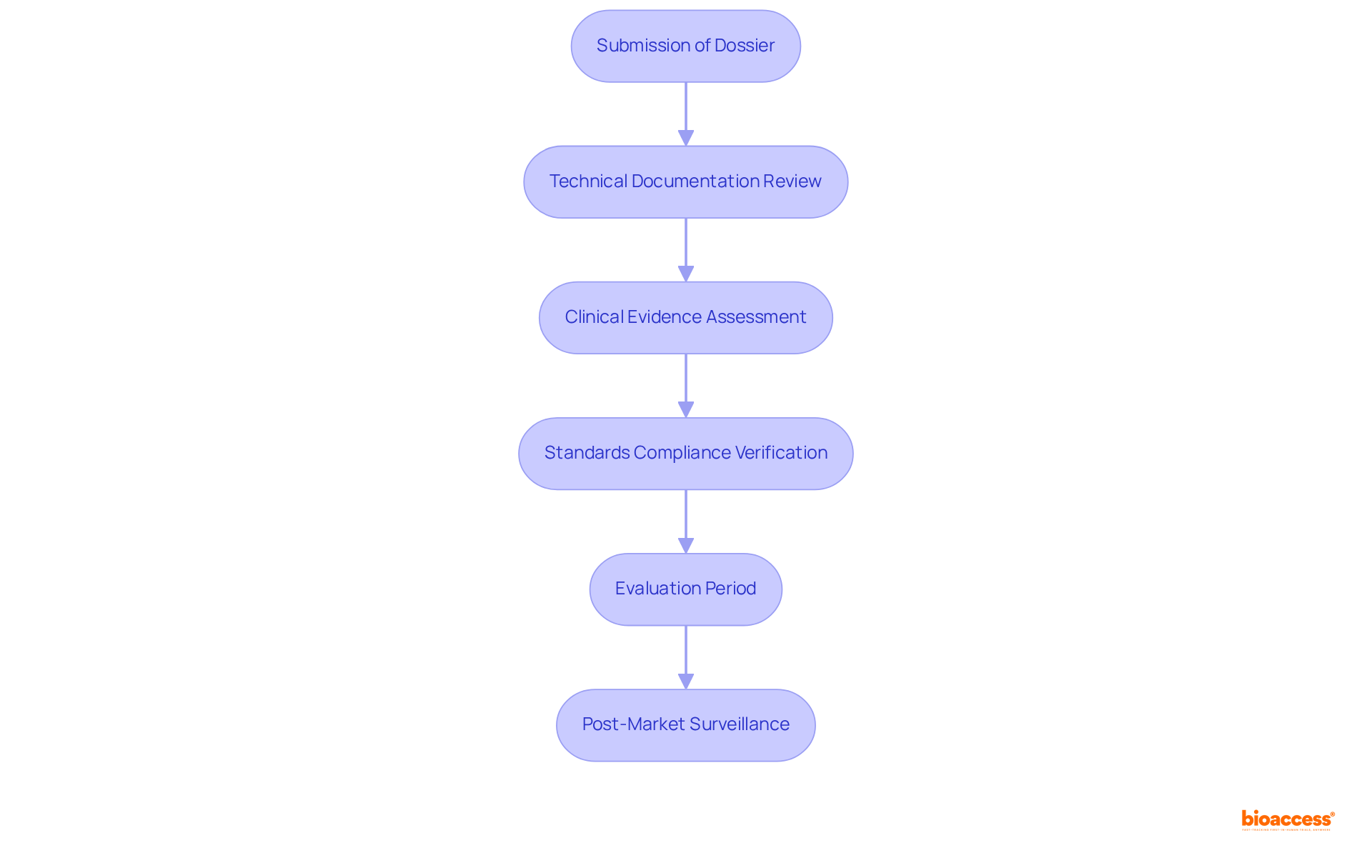
COFEPRIS plays a crucial role in the approval of medical products in Mexico, tasked with assessing their safety, effectiveness, and quality before they enter the market. The approval process involves a structured six-step assessment, requiring manufacturers to submit a comprehensive submission dossier. This dossier must include:
In 2025, the typical evaluation period for health equipment registration is anticipated to range from 3 to 8 months; however, utilizing an Authorized Third Party can expedite this to as little as 1 to 3 months. Furthermore, the regulatory agency conducts post-market surveillance, ensuring ongoing compliance with safety regulations and addressing any adverse events that may arise.
Understanding COFEPRIS's requirements and procedures is vital for manufacturers aiming to achieve swift approval and effective market entry in Mexico, especially regarding the medical device regulations in Mexico, as the healthcare equipment sector is projected to grow significantly, reaching an estimated value of $5.5 billion. Collaborating with experienced partners like bioaccess® can significantly enhance the likelihood of successfully navigating these regulatory pathways, as they offer tailored support in managing compliance, facilitating site feasibility studies, investigator selection, and streamlining the approval process through their comprehensive project management and reporting services.
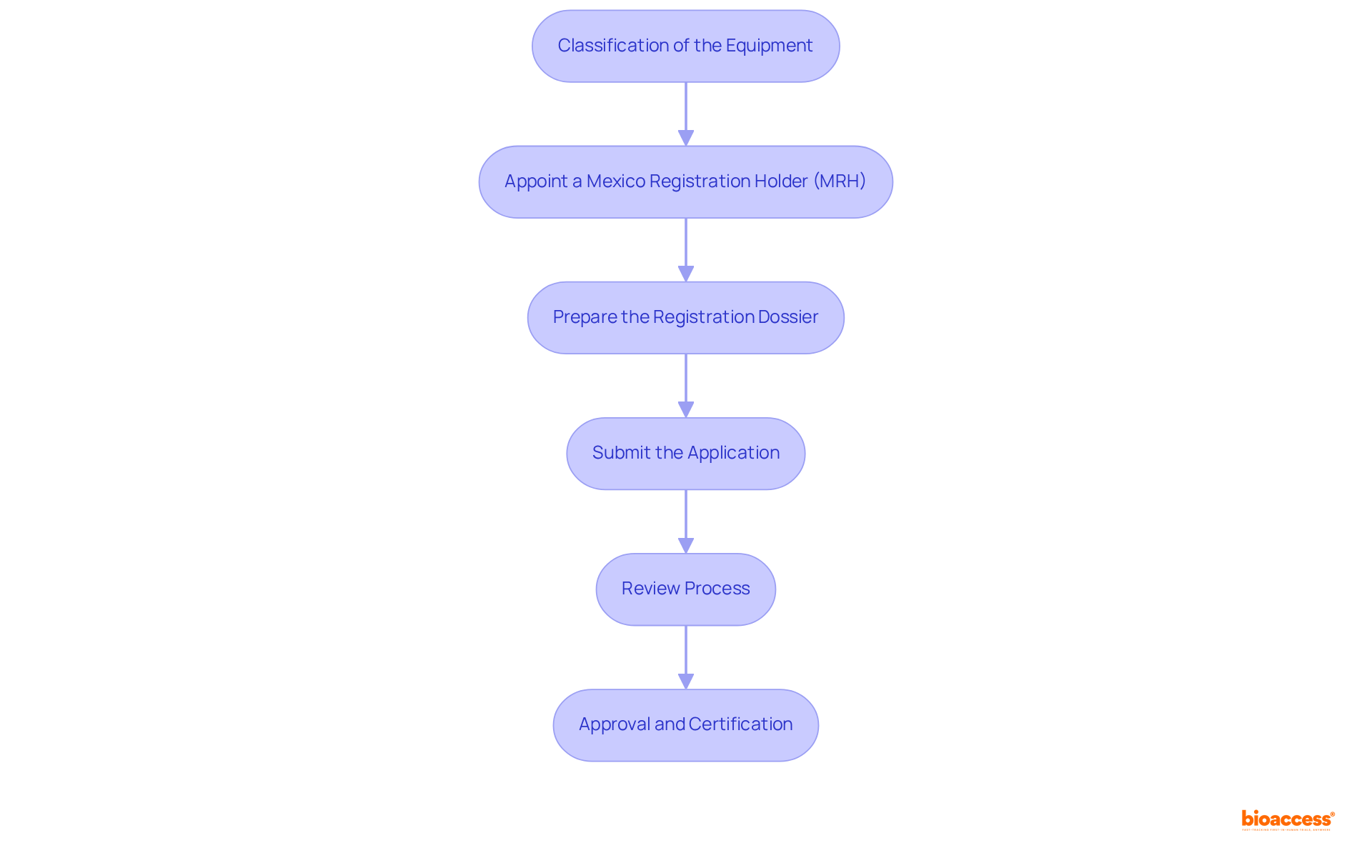
Classification of the Equipment: Start by determining the risk classification of your healthcare equipment, which is categorized into one of three levels: Class I (low risk), Class II (moderate risk), or Class III (high risk). This classification is critical as it dictates the application process and specific criteria. According to NOM-241-SSA1-2021, the classification of medical device regulations in Mexico is based on health risk, and medical devices are further divided into six families based on their function and purpose.
Appoint a Mexico Registration Holder (MRH): Designate a local representative responsible for managing the enrollment process and serving as the primary liaison with COFEPRIS. This local expertise is essential for navigating the complexities of medical device regulations in Mexico, as emphasized by industry leaders.
Prepare the Registration Dossier: Compile a comprehensive registration dossier that encompasses essential documentation, including technical specifications, clinical data, and proof of compliance with ISO 13485 or equivalent standards. For devices manufactured abroad, ensure that the dossier includes necessary legalizations and translations. This dossier must be meticulously organized to meet medical device regulations in Mexico, reflecting best practices discussed in the LATAM Medtech Leaders Podcast.
Submit the Application: Submit the complete registration dossier to the relevant authority, ensuring that all documents are translated into Spanish and formatted according to regulatory standards.
Review Process: The regulatory authority will conduct a thorough evaluation of the application, which may take between 3 to 8 months, depending on the product classification and submission completeness. Higher-risk instruments may face extended review periods. Notably, ethical approvals can be granted within 4-6 weeks, showcasing the efficiency of the process.
Approval and Certification: Upon successful evaluation, COFEPRIS will issue a certification document, permitting the product to be marketed in Mexico. It is crucial to monitor the validity of the enrollment, typically lasting 5 years, and to plan for timely renewals to ensure compliance. Additionally, consider the fast-track registration system available for products approved in the US, EU, Canada, Japan, and Switzerland, which can expedite the process. Leveraging the expertise of organizations like bioaccess® can further streamline this process, ensuring that your clinical trials and market entry strategies are effectively managed.
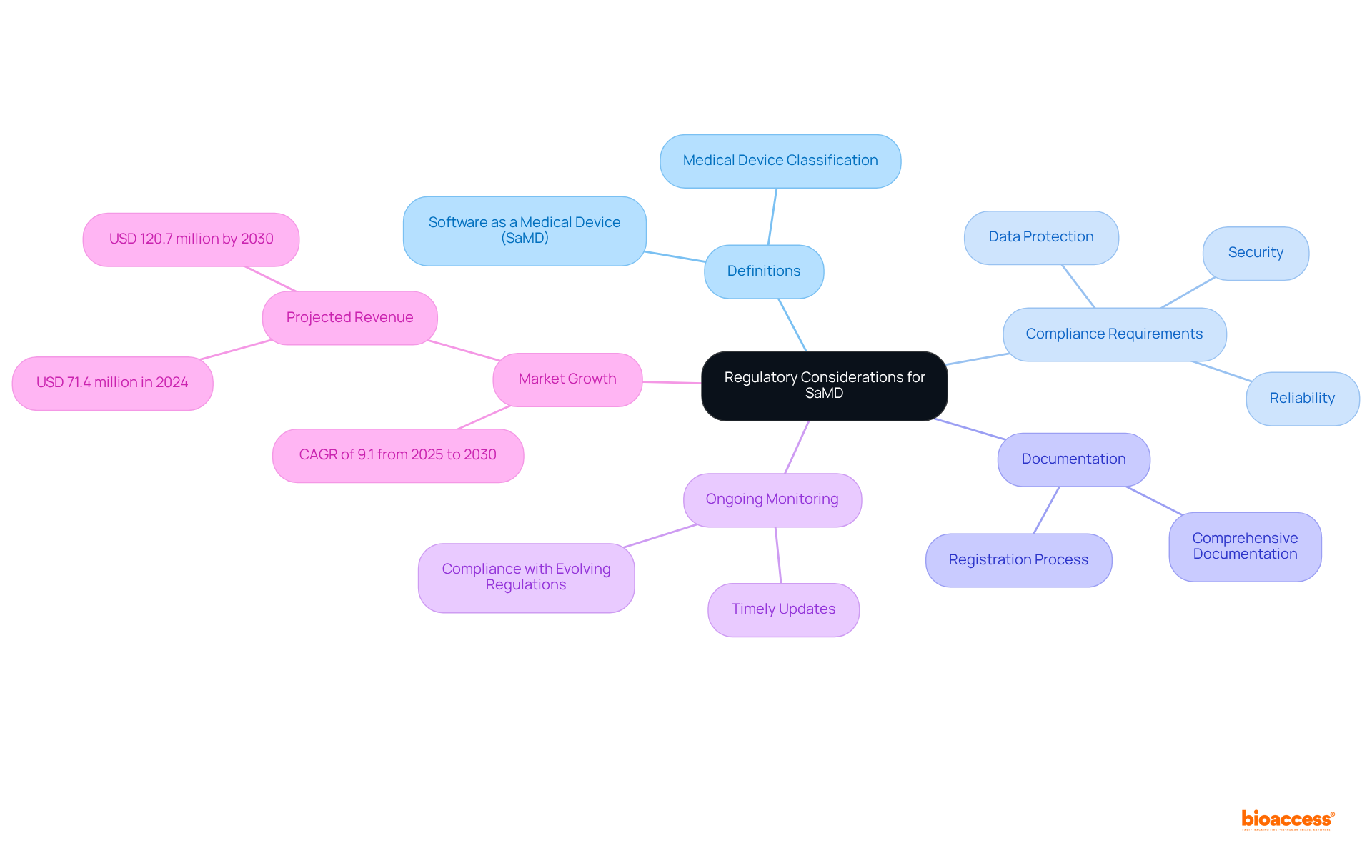
As digital health technologies advance, COFEPRIS has adapted its medical device regulations in Mexico to include Software as a Medical Device (SaMD). This classification is defined by the software's intended application and related risk level, reflecting the framework for conventional health equipment as outlined in medical device regulations Mexico.
Manufacturers must adhere to stringent technical requirements, including reliability, security, and data protection. Comprehensive documentation is essential to demonstrate compliance during the registration process, substantiating the software's safety and efficacy. Furthermore, ongoing monitoring and timely updates are critical to ensure continued compliance with evolving regulations.
Since the regulation of SaMD began on July 10, 2023, it is vital for manufacturers to stay informed about the medical device regulations in Mexico and any changes in this regulatory landscape to maintain their market position effectively.
Ana Criado, our Director of Regulatory Affairs, emphasizes the importance of understanding these regulations, given her extensive experience in regulatory affairs and her role in consulting for global companies. The market earnings for healthcare equipment regulatory affairs in Mexico are expected to reach USD 71.4 million in 2024 and USD 120.7 million by 2030, suggesting considerable growth potential.
As Fernanda Paro, a Regulatory Compliance Specialist, mentions, "SaMD is specifically defined as: 'Software used for one or more health-related purposes, which does not need to be integrated with the hardware of the health product to achieve its intended health purpose.'" This definition underscores the importance of understanding the regulatory framework surrounding SaMD within the context of medical device regulations Mexico.
Upon receiving approval from the regulatory authority, manufacturers are obligated to fulfill several post-approval responsibilities to ensure compliance and guarantee the ongoing safety and effectiveness of their medical products. These responsibilities encompass:
Renewal of Registration: Medical equipment registrations remain valid for five years and must be renewed prior to expiration. Manufacturers should proactively prepare for renewal by updating their documentation and confirming adherence to regulatory standards. The renewal process can now be conducted electronically, streamlining submissions and alleviating administrative burdens. The regulatory agency typically issues approvals for renewed Sanitary Registrations after a review period of 5-8 months, providing manufacturers with a clearer timeline for the renewal process.
Post-Market Surveillance: Ongoing monitoring of product performance in the marketplace is vital. Manufacturers are required to promptly report any adverse events or safety concerns to the regulatory authority, ensuring that potential risks are addressed within specified timeframes. Ana Criado emphasizes the necessity of establishing a robust reporting system to facilitate this process effectively.
Quality Management System (QMS): A compliant and effective QMS, adhering to ISO 13485 or equivalent standards, is crucial for maintaining the quality and safety of medical products. Manufacturers must provide evidence of an audited quality system, which is essential for demonstrating compliance with regulatory standards. This system should be regularly reviewed and updated to reflect any changes in regulatory requirements or operational practices, as underscored by Ana's extensive experience in regulatory affairs.
Labeling and Advertising Compliance: All labeling and advertising materials must conform to COFEPRIS regulations, which include specific language requirements and content accuracy. This ensures that consumers receive transparent and accurate information regarding the products. Ana's insights into effective communication strategies can assist manufacturers in fulfilling these requirements.
Technovigilance: Establishing a robust technovigilance system is critical for monitoring and documenting any concerns related to the safety and effectiveness of health products. This system should enable timely corrective actions in response to identified issues, thereby enhancing patient safety and regulatory compliance. The importance of patient safety and adherence to regulatory standards cannot be overstated, as it is essential for maintaining trust in healthcare technologies.
By adhering to these obligations, manufacturers can effectively navigate the complexities of the medical device regulations Mexico imposes, ensuring their products remain compliant and safe for users. With insights from experts like Ana Criado, who possesses extensive experience in regulatory affairs and biomedical engineering, manufacturers can gain a deeper understanding of the nuances of compliance in both Colombia and Mexico, further refining their market entry strategies.
Navigating the landscape of medical device regulations in Mexico is essential for manufacturers aiming to enter this rapidly growing market. The regulatory framework, primarily governed by COFEPRIS, outlines specific classifications and compliance requirements that dictate the path to market entry. Understanding these nuances ensures that manufacturers can effectively manage their products throughout the entire lifecycle, from registration to post-market obligations.
Key insights discussed include:
Additionally, the emergence of regulations governing Software as a Medical Device (SaMD) highlights the need for manufacturers to stay informed about evolving compliance requirements. The emphasis on post-registration obligations, including the renewal of registrations and ongoing surveillance, reinforces the critical nature of maintaining adherence to safety standards.
Ultimately, the significance of mastering medical device regulations in Mexico cannot be overstated. As the market continues to expand, manufacturers must prioritize compliance to safeguard patient safety and ensure their products are effectively integrated into the healthcare system. Engaging with experienced partners and staying abreast of regulatory changes will not only enhance the likelihood of successful market entry but also contribute to the overall advancement of healthcare technologies in Mexico.
What governs the medical device regulations in Mexico?
The medical device regulations in Mexico are governed by the General Health Law (Ley General de Salud) and enforced by the Federal Commission for the Protection against Sanitary Risk (COFEPRIS).
How are medical devices classified in Mexico?
Medical devices in Mexico are classified into three risk categories: Class I (low-risk), Class II (moderate-risk), and Class III (high-risk).
What impact does the classification of medical devices have?
The classification impacts the entry process, documentation requirements, and timelines for market access.
What new mandates are set to begin in 2025 for low-risk medical products?
Starting in 2025, low-risk products will require sanitary registration and compliance with Good Manufacturing Practices (GMP).
What percentage of healthcare instruments sold in Mexico are imports?
Nearly 90% of healthcare instruments sold in Mexico are imports.
What is the projected market value for the healthcare equipment sector in Mexico?
The healthcare equipment sector in Mexico is projected to reach a value of US$5.5 billion.
What role does COFEPRIS play in medical device registration?
COFEPRIS is responsible for assessing the safety, effectiveness, and quality of medical products before they enter the market.
What does the approval process by COFEPRIS involve?
The approval process involves a structured six-step assessment, requiring manufacturers to submit a comprehensive submission dossier that includes technical documentation, clinical evidence, and proof of adherence to relevant standards.
What is the anticipated evaluation period for health equipment registration in 2025?
The typical evaluation period for health equipment registration in 2025 is expected to range from 3 to 8 months.
How can the evaluation period be expedited?
Utilizing an Authorized Third Party can expedite the evaluation period to as little as 1 to 3 months.
What ongoing responsibilities does COFEPRIS have after market entry?
COFEPRIS conducts post-market surveillance to ensure ongoing compliance with safety regulations and address any adverse events.
Why is it important for manufacturers to understand COFEPRIS's requirements?
Understanding COFEPRIS's requirements and procedures is vital for manufacturers aiming to achieve swift approval and effective market entry in Mexico.