


This article provides essential compliance insights critical for mastering medical device software development. It emphasizes the necessity of understanding software classifications and adhering to regulatory standards. By detailing various categories of medical software and the regulatory frameworks, including FDA and ISO standards, it highlights best practices within the software development lifecycle. These elements are crucial for ensuring safety, effectiveness, and compliance in healthcare technology.
The landscape of medical device software development is rapidly evolving, driven by the increasing integration of technology in healthcare and the pressing need for compliance with stringent regulations. Developers face the critical task of navigating complex classifications and regulatory standards, such as those set forth by the FDA and INVIMA, to ensure their products not only meet safety requirements but also enhance patient outcomes. With the stakes so high, developers must effectively balance innovation with compliance.
What best practices can they adopt to streamline the software development lifecycle? This question is central to understanding the future of Medtech and the role of bioaccess in addressing these key challenges.
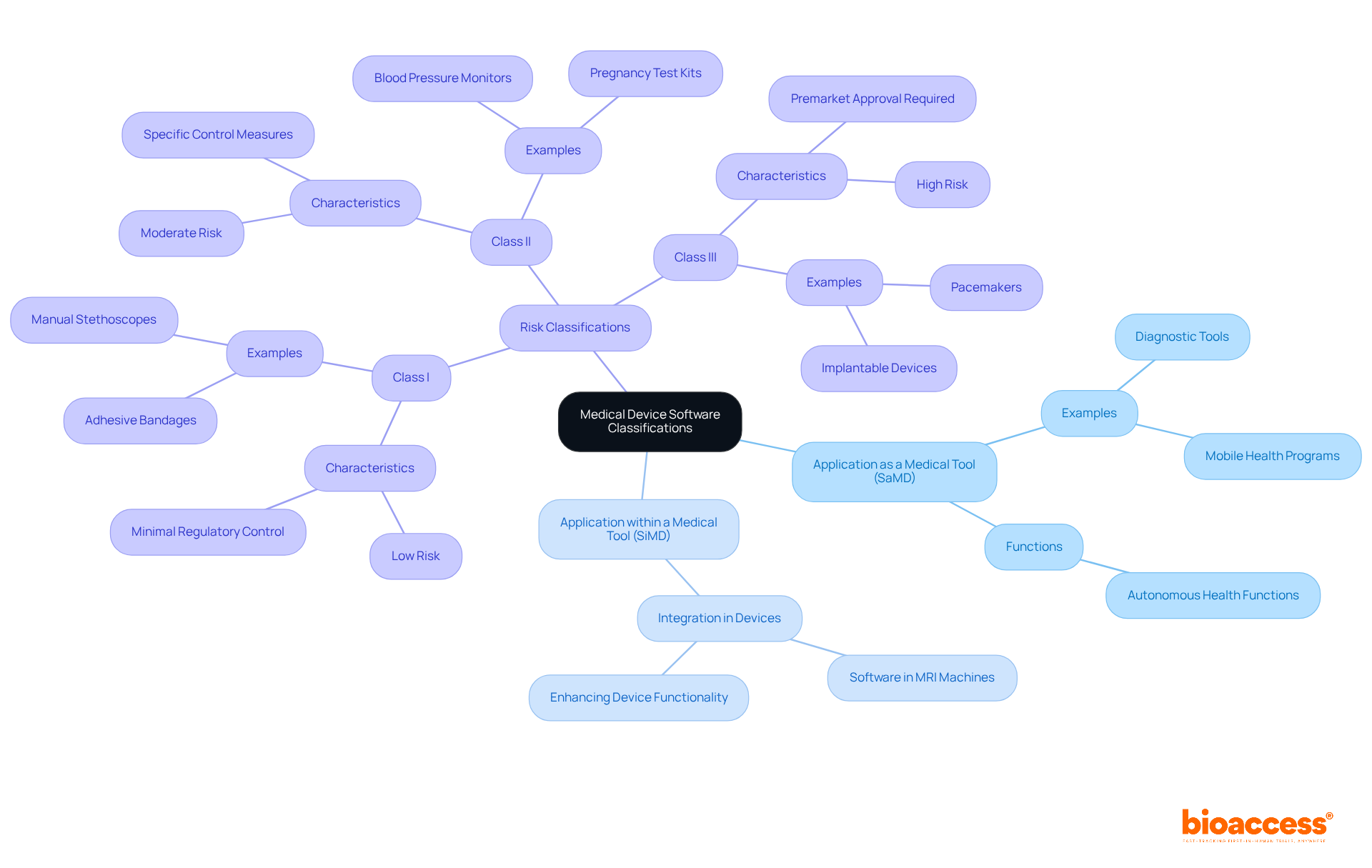
Medical application programs can be classified into two primary categories: Application as a Medical Tool (SaMD) and Application within a Medical Tool (SiMD). SaMD refers to applications designed to autonomously perform health-related functions, such as diagnostic tools or mobile health programs. Conversely, SiMD is integrated within healthcare devices, enhancing or managing their functionality, akin to software embedded in an MRI machine.
In Colombia, the regulatory framework for health products is overseen by INVIMA (Colombia National Food and Drug Surveillance Institute), which plays a vital role in ensuring the safety, effectiveness, and quality of health products. INVIMA is responsible for the examination and oversight of health product promotion and production, including medical equipment. The organization is classified as a Level 4 health authority by the Pan American Health Organization/World Health Organization, underscoring its competence in health regulation functions.
Healthcare software categories are typically determined by the associated risk levels. The FDA classifies these into three distinct classes:
Understanding these classifications, along with the oversight provided by INVIMA, is essential for developers in medical device software development to ensure compliance with necessary regulations and facilitate smoother approval processes. Experts emphasize that effectively navigating these classifications can significantly enhance patient safety and regulatory compliance.
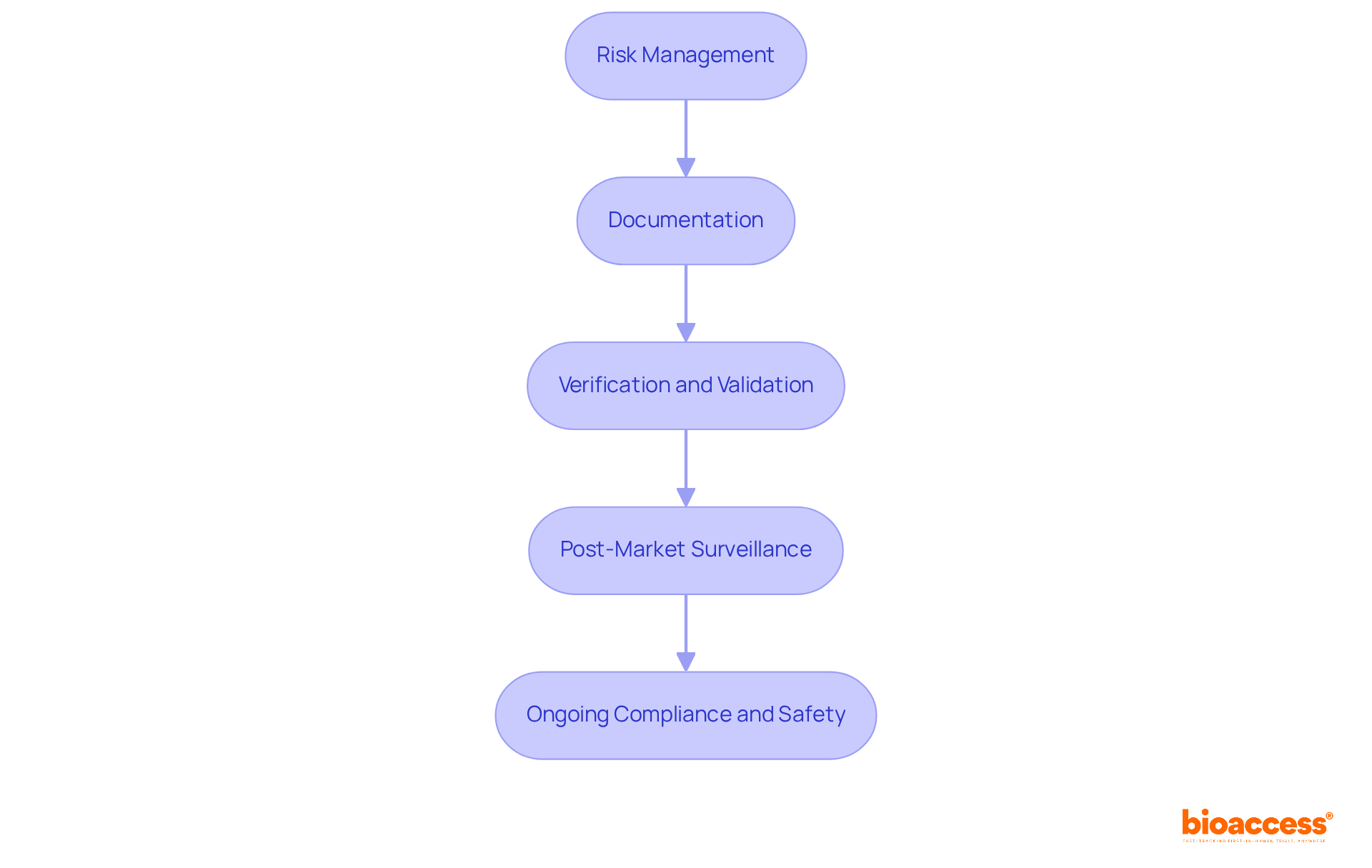
Adhering to regulatory standards like the FDA's Quality System Regulations (QSR) and the ISO 13485 standard is crucial for medical device software development. These regulations are designed to ensure that products are developed, manufactured, and maintained to meet stringent safety and quality requirements.
Key compliance steps include:
Following these guidelines and quality assurance measures not only guarantees adherence but also improves the safety and efficiency of health technology for end-users. Recent trends suggest that the adoption rate of ISO 13485 among healthcare product manufacturers is steadily rising, reflecting a growing commitment to quality management systems that align with international standards. Moreover, updates to the FDA's QSR highlight the necessity for a thorough approach to software development, strengthening the significance of these compliance measures in today's oversight environment.
Specialists such as Ana Criado and Katherine Ruiz, who possess significant expertise in compliance matters and biomedical engineering, can offer important perspectives on managing the intricacies of adherence in the healthcare equipment industry. By addressing common pitfalls in compliance, such as inadequate documentation or insufficient risk management processes, developers can better ensure their products meet legal requirements and are safe for end-users.
To ensure the successful development of medical device applications, it is essential to implement best practices in medical device software development throughout the Software Development Lifecycle (SDLC). Key practices include:
Agile Methodology: Adopting an agile approach facilitates iterative development, allowing teams to respond swiftly to changes and incorporate feedback throughout the process. Organizations that embrace agile practices report a 30% increase in project efficiency and a 50% improvement in employee engagement, according to a Harvard Business Review survey.
Cross-Functional Cooperation: Encouraging teamwork among programmers, compliance specialists, and clinical researchers enhances comprehension of requirements and greatly boosts product quality. Companies utilizing cross-functional teams are 60% more likely to achieve their innovation goals, and those that prioritize collaboration see a 50% increase in productivity. Furthermore, organizations with high collaboration levels are five times more likely to deliver superior performance.
Automated Testing: Utilizing automated testing tools can significantly cut down the time required for validation and verification, ensuring that applications meet quality standards prior to release. This method not only simplifies the testing process but also improves adherence to legal requirements.
Continuous Integration and Deployment (CI/CD): Utilizing CI/CD practices enables frequent updates and enhancements to the application, ensuring it remains compliant with evolving regulations and user needs. Organizations that adopt CI/CD methodologies can reduce product development time by up to 25%, allowing for quicker responses to market demands.
While these practices provide substantial advantages, it is crucial to recognize the difficulties of applying Agile in healthcare product development, such as aligning practices with regulatory requirements and maintaining documentation for compliance. By integrating these best practices into the SDLC, developers can significantly enhance the efficiency and effectiveness of their processes in medical device software development, ultimately leading to higher quality products and improved patient outcomes.

Mastering the intricacies of medical device software development is essential for ensuring compliance and enhancing patient safety. The classifications of software, whether as a standalone medical tool or integrated within medical devices, highlight the diverse landscape developers must navigate. Understanding the regulatory frameworks, particularly those established by entities like INVIMA and the FDA, is critical for achieving successful product approval and market entry.
Key insights from the article emphasize the importance of adhering to regulatory standards such as the FDA's Quality System Regulations and ISO 13485. These frameworks not only guide developers in risk management, documentation, and testing but also reinforce the necessity of maintaining high-quality and safe health technologies. Implementing best practices throughout the Software Development Lifecycle—such as:
can significantly enhance product development efficiency and regulatory compliance.
In light of the evolving landscape of medical device software development, embracing these compliance measures and best practices is not merely a regulatory obligation but a vital component of delivering safe and effective healthcare solutions. Developers are encouraged to stay informed about current trends and regulatory updates, ensuring that their products not only meet legal standards but also contribute positively to patient outcomes. By prioritizing compliance and quality assurance, the healthcare industry can better serve its mission of safeguarding public health.
What are the two primary categories of medical application programs?
The two primary categories are Software as a Medical Device (SaMD), which performs health-related functions autonomously, and Software within a Medical Device (SiMD), which is integrated within healthcare devices to enhance their functionality.
What is SaMD?
SaMD refers to applications designed to autonomously perform health-related functions, such as diagnostic tools or mobile health programs.
What is SiMD?
SiMD is software that is integrated within healthcare devices, enhancing or managing their functionality, similar to software embedded in devices like MRI machines.
Who oversees the regulatory framework for health products in Colombia?
The regulatory framework for health products in Colombia is overseen by INVIMA (Colombia National Food and Drug Surveillance Institute).
What is the role of INVIMA?
INVIMA ensures the safety, effectiveness, and quality of health products, including medical equipment, and is responsible for the examination and oversight of health product promotion and production.
How does the FDA classify healthcare software?
The FDA classifies healthcare software into three distinct classes based on associated risk levels: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What defines Class I medical devices?
Class I medical devices are characterized by low risk and are subject to minimal regulatory control, with approximately 47% of medical devices falling into this category. Examples include general wellness applications and manual stethoscopes.
What is required for Class II medical devices?
Class II medical devices, which account for around 43% of healthcare instruments, require adherence to specific control measures and must undergo the 510(k) premarket notification process to demonstrate substantial equivalence to existing products.
What are Class III medical devices?
Class III medical devices represent high risk and mandate premarket approval due to significant potential risks to patients. This class includes tools that manage life-supporting devices and comprises approximately 10% of healthcare instruments.
Why is understanding these classifications important for developers?
Understanding these classifications, along with the oversight provided by INVIMA, is essential for developers in medical device software development to ensure compliance with necessary regulations and facilitate smoother approval processes, ultimately enhancing patient safety and regulatory compliance.