


Phase 0 clinical trials, commonly referred to as exploratory IND studies, serve a vital purpose in the realm of drug development. These trials are meticulously designed to gather preliminary data on a drug's pharmacokinetics and pharmacodynamics by administering subtherapeutic doses to a select group of participants. Their significance cannot be overstated; they play a crucial role in refining the drug development process.
By identifying promising candidates for further testing, these trials effectively minimize the risks and costs associated with bringing new drugs to market. In this context, the emphasis on Phase 0 trials highlights their indispensable contribution to the overall success of clinical research.
Phase 0 clinical trials represent a pivotal yet often overlooked stage in the drug development process, serving as the crucial first step in understanding how new substances interact with human biology. These exploratory studies not only provide invaluable insights into pharmacokinetics and pharmacodynamics but also streamline the development pipeline by identifying promising candidates early on.
However, as the number of these trials continues to grow, so do the ethical complexities surrounding informed consent and risk assessment. How can researchers balance the urgency of innovation with the imperative of participant safety in this unique landscape? This question underscores the importance of navigating these challenges with both diligence and expertise.
Stage 0 clinical studies, also referred to as exploratory investigational new substance (IND) studies, mark the initial phase of clinical research. These studies typically involve administering subtherapeutic doses of a medication to a limited group of human volunteers, often numbering fewer than 15 participants. The primary objective of Stage 0 studies is to collect preliminary data regarding a substance's behavior in humans, with a particular focus on its pharmacokinetics (PK) and pharmacodynamics (PD).
Unlike traditional clinical studies that assess therapeutic effects, these early investigations aim to confirm the compound's interaction with its intended biological target. This initial evaluation is crucial, as it assists in refining the medication development process by identifying promising candidates for further testing in Stage I evaluations. Consequently, Stage 0 experiments can significantly reduce both the time and costs associated with drug development.
As of 2025, the number of phase 0 clinical trials conducted globally continues to rise, reflecting an increasing recognition of their importance within the pharmaceutical sector. The insights gained from these studies not only enhance the efficiency of drug development but also uphold the ethical imperative of ensuring that only the most promising compounds advance to subsequent testing phases.
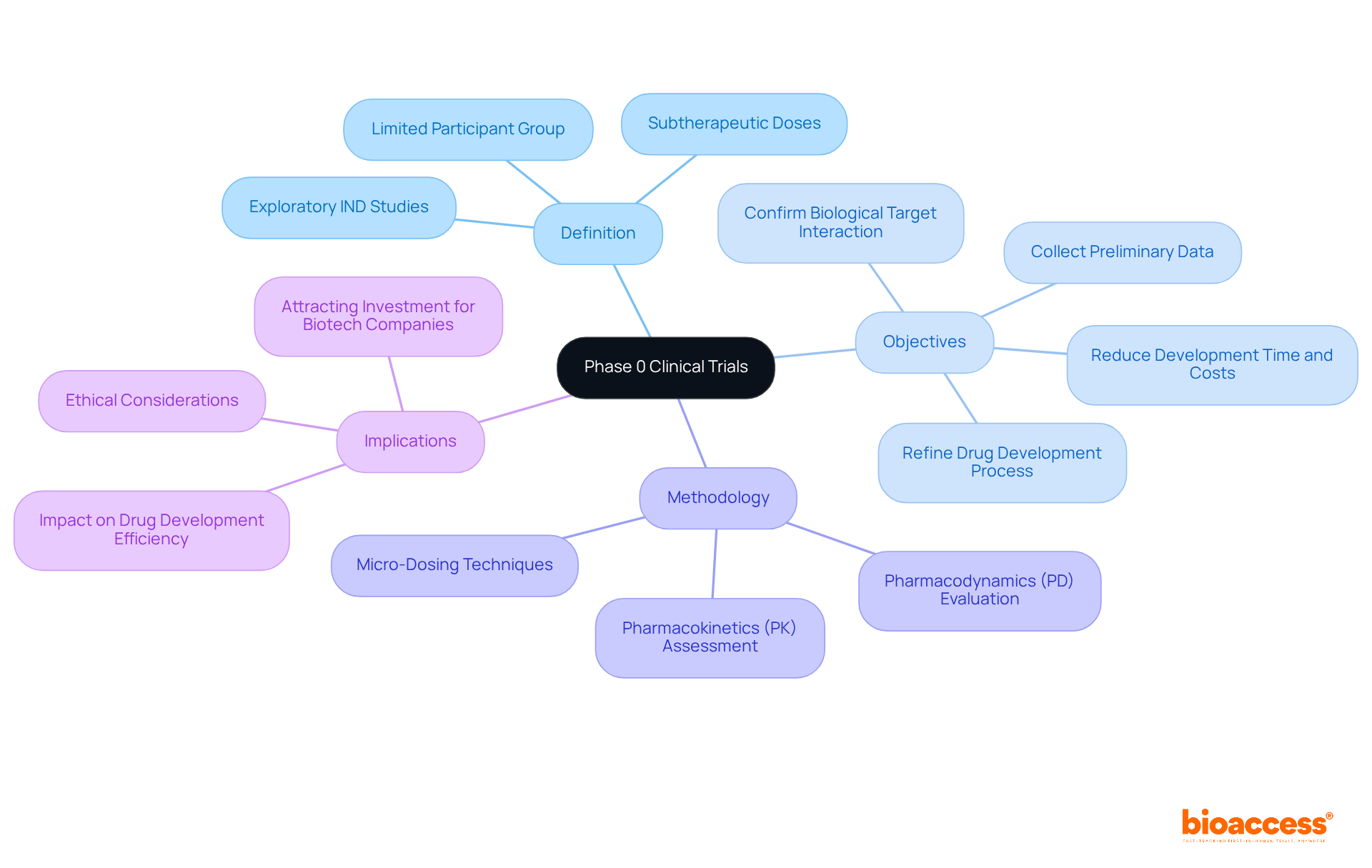
Phase 0 trials serve several critical objectives in the early stages of drug development:
Evaluating Substance Behavior: These studies aim to understand how a substance interacts with its target, providing initial insights into its pharmacokinetics and pharmacodynamics. Recent studies suggest that a considerable proportion of evaluations in phase 0 clinical trials examine medication behavior, highlighting their significance in the clinical study environment.
Identifying Optimal Dosing: By utilizing microdoses—typically 1/100th of the pharmacologically active dose—researchers can establish a safe initial dose for future studies, minimizing risk to volunteers.
Streamlining Medication Development: Stage 0 experiments accelerate the medication development process by eliminating ineffective compounds early, thereby conserving time and resources.
The types of Phase 0 trials include:
As pointed out by the American Cancer Society medical and editorial content team, 'Phase 0 clinical trials differ from the other levels of clinical research,' highlighting their distinctive function in the medication development process. Integrating insights from these experiments can greatly improve the comprehension of medication behavior and guide future research paths.
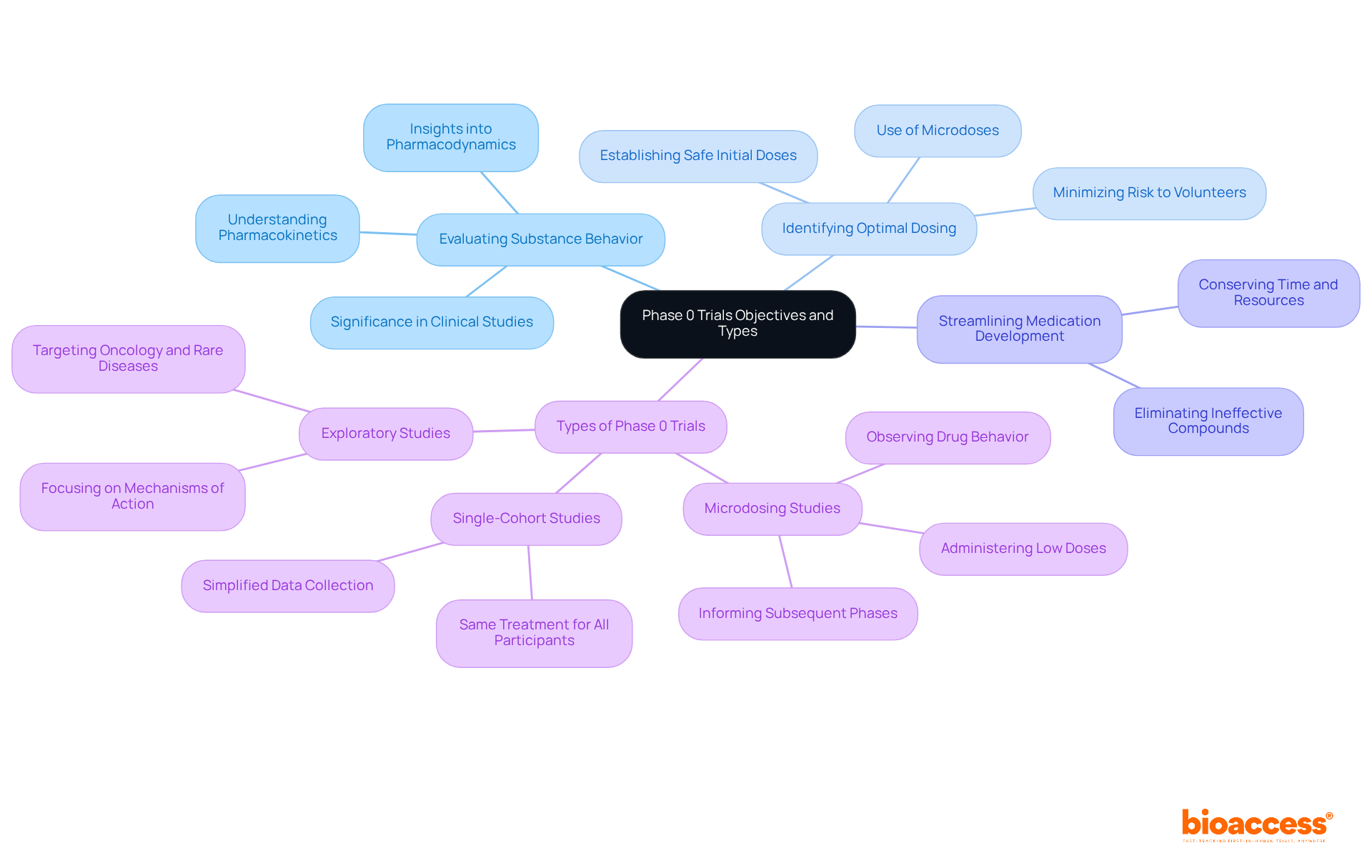
Dose selection in a phase 0 clinical trial is a crucial process that determines the suitable microdose for human administration. Typically, this dose is set at 1/100th of the pharmacologically active dose or 1/50th of the no observed adverse effect level (NOAEL) established in preclinical studies. The aim is to guarantee that the dose is adequately low to reduce risk while still providing valuable insights into the substance's behavior in humans. The maximum dose in Stage 0 studies may not exceed 100 micrograms.
Preclinical testing requirements for Phase 0 trials include:
These preclinical assessments are essential for verifying the drug's appropriateness for human testing and guiding the design of the phase 0 clinical trial. It is important to note that approximately 40% of Stage I failures are attributed to unacceptable pharmacokinetic (PK) profiles, underscoring the necessity of thorough preclinical testing. As Bob Wesdorp mentioned, "Preclinical work guarantees the safety of your Stage 0 study." Furthermore, Stage 0 studies must comply with ethical standards like respect for individuals, beneficence, and fairness to safeguard participant welfare.
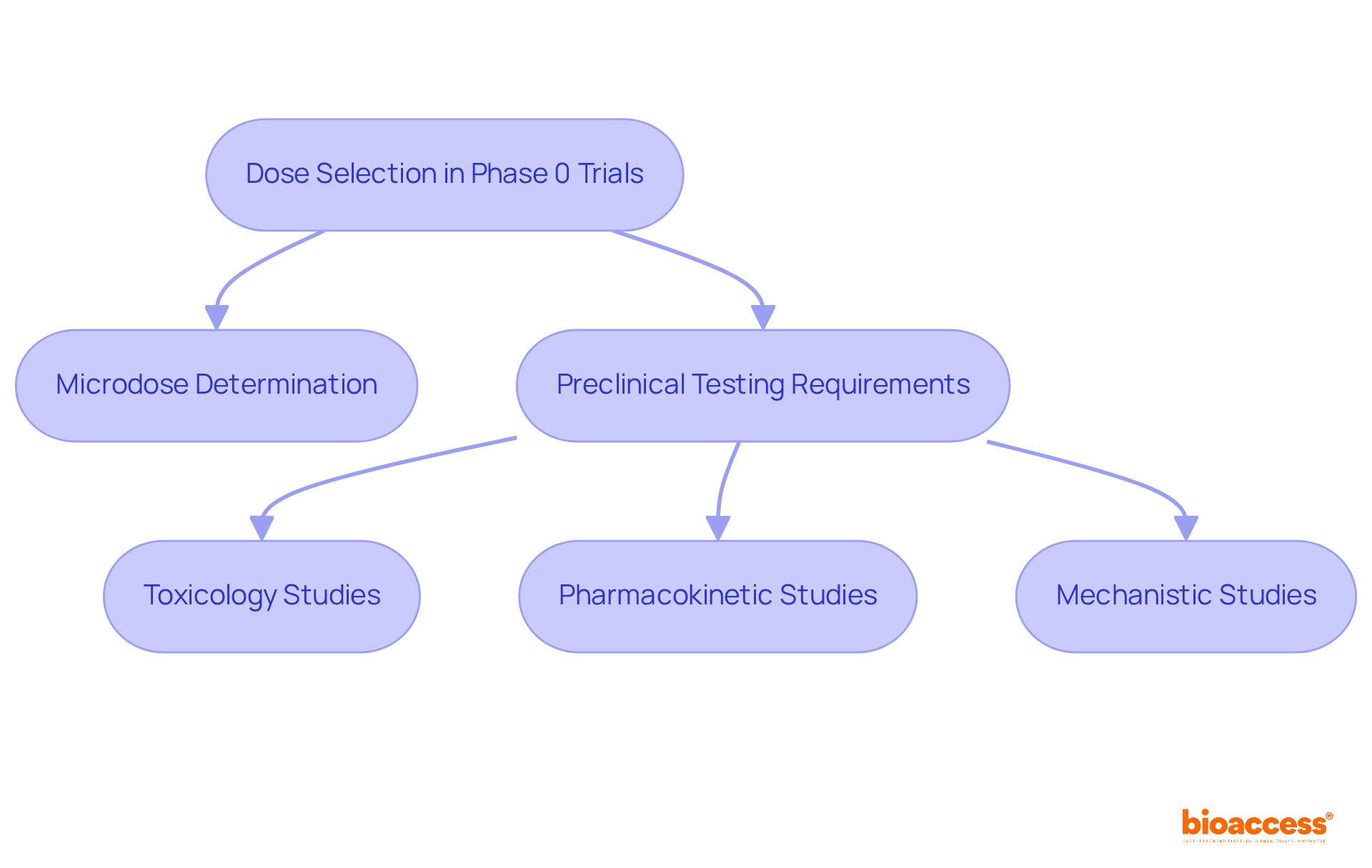
The ethical issues presented by phase 0 clinical trials warrant careful consideration. Informed Consent is paramount; participants must be thoroughly informed about the study's nature, including the absence of therapeutic intent and potential risks. It is crucial to ensure that consent is acquired willingly and without pressure, as research indicates that only approximately 60% of individuals in preliminary studies feel completely informed about the study's aims and hazards. Typically, initial studies involve 10 to 15 individuals to gather preliminary data on pharmacokinetics and pharmacodynamics.
Another critical aspect is the Risk vs. Benefit evaluation. Since Phase 0 trials do not aim to provide therapeutic advantages, researchers must justify the risks associated with administering a microdose to subjects. This requires a comprehensive assessment of potential benefits for future patients against the risks for current participants, ensuring that the scientific significance outweighs the ethical considerations.
The Vulnerability of Individuals involved in these trials also demands special attention. It is essential to ensure that vulnerable populations are not exploited and that their rights and welfare are safeguarded. Ethical dilemmas, such as challenges in informed consent and the use of placebos, must be navigated with care to uphold the rights of those participating in the research.
Lastly, Regulatory Compliance is vital. Adhering to ethical guidelines and regulations, such as the Declaration of Helsinki, is essential for maintaining the integrity of the research and protecting participants. Furthermore, the responsibility for transparency in medication studies rests with sponsors, researchers, and regulatory bodies, ensuring that the study adheres to established ethical standards and fosters confidence in the clinical research process.
Addressing these ethical considerations is crucial for the successful execution of phase 0 clinical trials and for sustaining public confidence in clinical research. Well-conducted clinical drug trials are widely recognized as the primary source of evidence regarding the safety and effectiveness of medical interventions, highlighting the importance of transparency and participant education in advancing medical research.
Phase 0 clinical trials represent a pivotal starting point in the drug development process, concentrating on the initial evaluation of new substances in humans. By administering microdoses to a small cohort of participants, these studies aim to gather critical data on pharmacokinetics and pharmacodynamics, ultimately guiding the selection of promising candidates for further testing. The significance of Phase 0 trials lies in their capacity to streamline the medication development process, reduce costs, and uphold ethical standards.
The article highlights several key objectives of Phase 0 trials, including:
Various types of Phase 0 trials, such as microdosing and exploratory studies, serve distinct purposes that contribute to a deeper understanding of drug interactions and mechanisms. Furthermore, the importance of rigorous preclinical testing and ethical considerations, such as informed consent and risk assessment, underscores the complexities involved in conducting these trials responsibly.
In conclusion, mastering Phase 0 clinical trials is essential for advancing drug development while ensuring participant safety and ethical integrity. As the pharmaceutical industry continues to recognize the value of these exploratory studies, stakeholders must remain committed to:
By embracing these principles, the path toward innovative and effective therapies can be significantly enhanced, ultimately benefiting both researchers and patients alike.
What are Phase 0 clinical trials?
Phase 0 clinical trials, also known as exploratory investigational new substance (IND) studies, are the initial phase of clinical research that involves administering subtherapeutic doses of a medication to a limited group of human volunteers, typically fewer than 15 participants.
What is the primary purpose of Phase 0 clinical trials?
The primary purpose of Phase 0 clinical trials is to collect preliminary data on a substance's behavior in humans, focusing on its pharmacokinetics (PK) and pharmacodynamics (PD), rather than assessing therapeutic effects.
How do Phase 0 trials differ from traditional clinical studies?
Unlike traditional clinical studies that evaluate therapeutic effects, Phase 0 trials aim to confirm a compound's interaction with its intended biological target and refine the medication development process.
What benefits do Phase 0 clinical trials provide in drug development?
Phase 0 clinical trials help identify promising candidates for further testing in Stage I evaluations, significantly reducing the time and costs associated with drug development.
What is the trend regarding Phase 0 clinical trials as of 2025?
As of 2025, the number of Phase 0 clinical trials conducted globally is increasing, indicating a growing recognition of their importance in the pharmaceutical sector.
How do Phase 0 trials contribute to ethical drug development?
The insights gained from Phase 0 trials enhance the efficiency of drug development and ensure that only the most promising compounds advance to subsequent testing phases, aligning with ethical imperatives in the field.