Overview
This article delineates key strategies for the successful execution of Phase 3 trials, underscoring the significance of:
- Effective design methodologies
- Recruitment strategies
- Data analysis
It asserts that meticulous planning, which includes:
- Robust statistical methods
- Adherence to regulatory standards
is vital for ensuring the credibility of the trials and ultimately facilitating the approval of new therapies.
Introduction
Phase 3 clinical trials represent a pivotal juncture in the journey of drug development, serving as the final evaluation stage before a treatment can secure regulatory approval. These trials not only confirm the efficacy and safety of new interventions but also navigate a complex landscape of statistical methodologies, recruitment strategies, and regulatory challenges. Despite their significance, many trials falter due to common pitfalls in execution.
Thus, what are the key strategies that can ensure success in Phase 3 trials, and how can researchers effectively overcome the obstacles they face?
Define Phase 3 Trials: Importance and Objectives
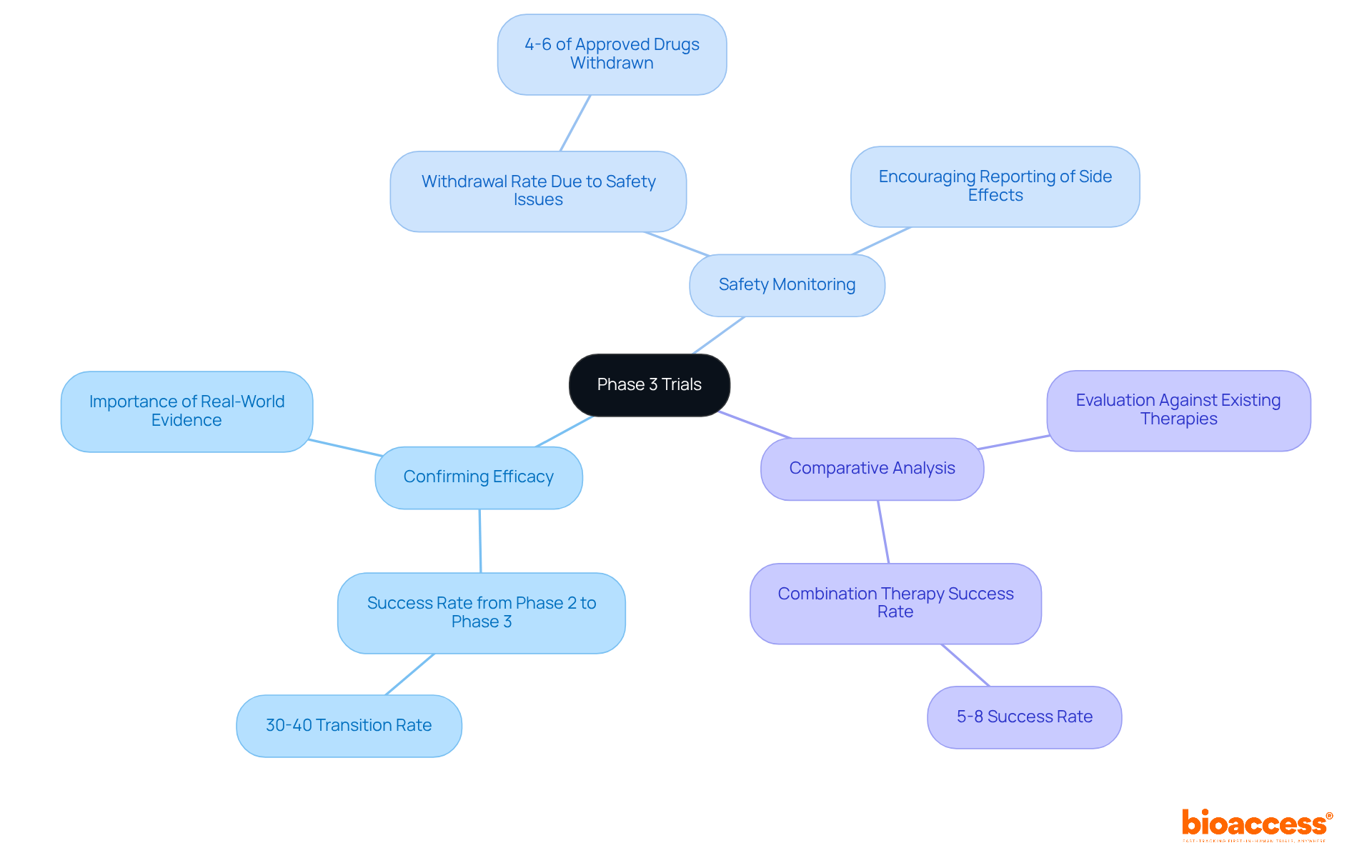
Phase 3 trials represent the final step of clinical evaluation before a medication is submitted for regulatory endorsement. These phase 3 trials typically involve extensive participant groups, ranging from hundreds to thousands, and are designed to confirm the effectiveness of an intervention, monitor side effects, and compare the new intervention with standard or placebo alternatives. The primary objectives of Phase 3 trials include:
- Confirming Efficacy: Establishing that the new treatment is effective across a broader population, which is crucial as only about 30%-40% of drugs transition successfully from Phase 2 to Phase 3.
- Safety Monitoring: Safety monitoring during phase 3 trials involves identifying adverse effects that may not have been evident in earlier phases. Approximately 4%-6% of approved drugs are withdrawn from the market due to safety issues, highlighting the necessity of rigorous safety monitoring during phase 3 trials.
- Comparative Analysis: Comparative analysis involves evaluating how the new treatment performs against existing therapies, especially during phase 3 trials, which is essential for demonstrating its value in the market.
To ensure the success of Stage 3 assessments, bioaccess provides comprehensive clinical study management services, including feasibility analyses, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These services are vital for navigating the complexities of regulatory approval, with around 50%-60% of drugs entering phase 3 trials ultimately receiving FDA approval. The phase 3 trials serve as a cornerstone of clinical research, ensuring that new therapies are both effective and safe for public use, while also contributing to local economies through job creation and healthcare improvements.
Explore Design Methodologies: Statistical Approaches and Trial Structures
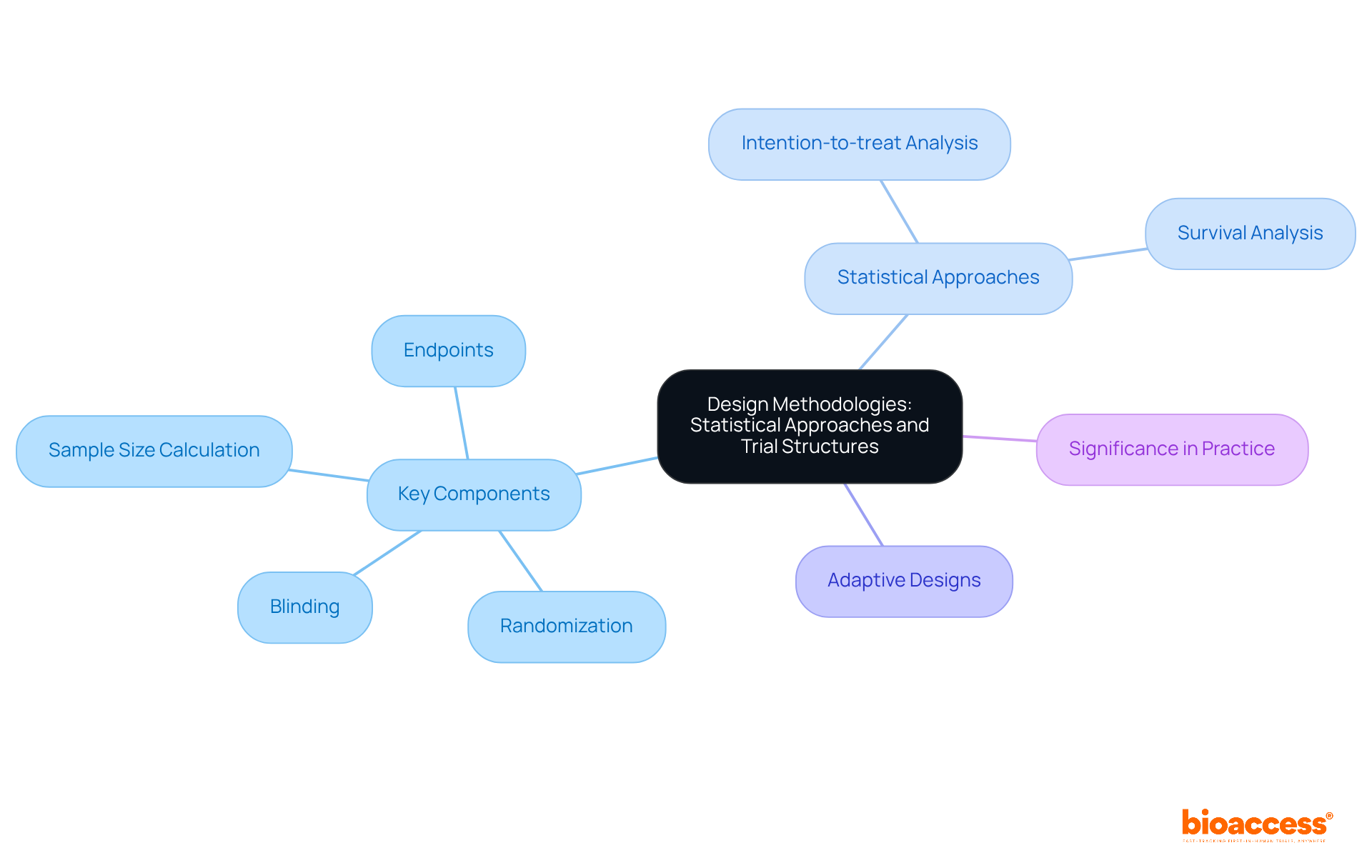
Creating a third stage study necessitates a meticulous focus on statistical methods and study frameworks. Key components include:
- Randomization: Participants are randomly assigned to treatment or control groups, effectively minimizing bias and enhancing the validity of the results. This process is essential, as randomization significantly influences the statistical results of phase 3 trials, ensuring that the groups are comparable and that the observed effects can be linked to the intervention. As noted by Leila Janani, contemporary studies benefit from innovative methods such as adaptive designs, which allow for scheduled adjustments based on gathered information.
- Blinding: Implementing double-blind designs is crucial, as it ensures that neither participants nor researchers are aware of who is receiving the treatment. This method reduces the likelihood of placebo effects and observer bias, thereby enhancing the validity of the findings.
- Sample Size Calculation: Accurately determining the number of participants required is vital for achieving statistically significant results. This calculation must account for the expected effect size, variability, and desired power of the study.
- Endpoints: Clearly defining primary and secondary endpoints is necessary to measure the study's success effectively. These endpoints guide the analysis and interpretation of the data collected.
Common statistical approaches employed in Phase 3 trials include:
- Intention-to-treat Analysis: This method analyzes participants based on their original group assignment, regardless of whether they completed the trial. It preserves the advantages of randomization and helps mitigate biases that could arise from differential dropout rates.
- Survival Analysis: Techniques such as Kaplan-Meier curves are utilized to assess time-to-event data, providing insights into the duration of treatment effects and overall survival rates.
Furthermore, the TEAM III study exemplifies the significance of these methodologies in practice, showcasing how adaptive designs can enhance study efficiency and outcomes. These methodologies are essential in ensuring that testing results are credible and can effectively support regulatory submissions, ultimately facilitating the advancement of innovative therapies.
Implement Recruitment Strategies: Navigating Regulatory Challenges
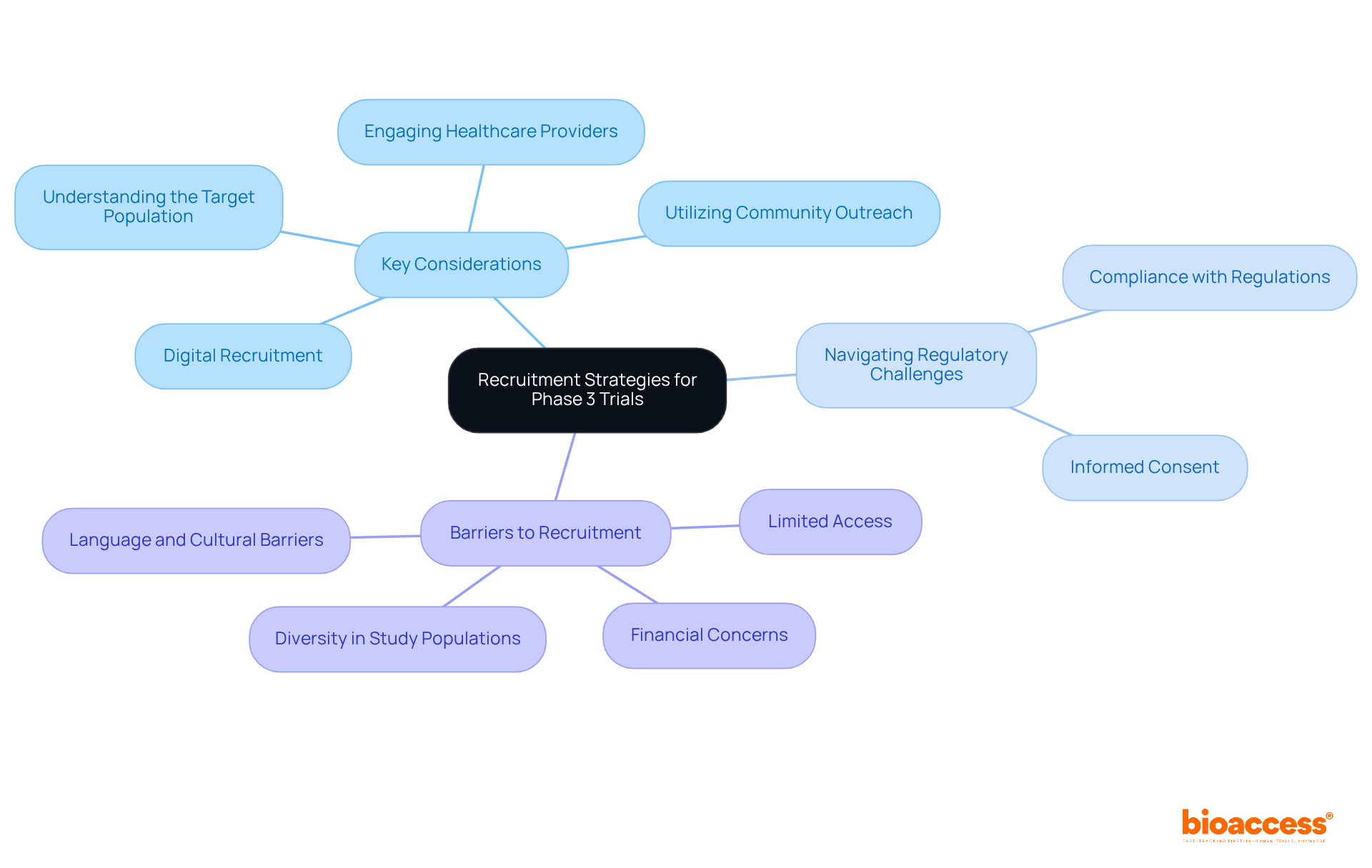
Effective recruitment strategies are crucial for the success of phase 3 trials. Key considerations include:
- Understanding the Target Population: Accurately identifying and characterizing the patient population that meets the trial criteria is crucial for ensuring relevant and applicable results.
- Engaging Healthcare Providers: Establishing strong relationships with physicians is vital, as they can refer eligible patients and enhance recruitment efforts significantly.
- Utilizing Community Outreach: Leveraging local community networks and patient advocacy groups can effectively raise awareness about the study, fostering trust and participation.
- Digital Recruitment: Utilizing social media and online platforms enables researchers to connect with a wider audience, enhancing the visibility of the study among potential participants.
bioaccess® offers accelerated patient recruitment and site activation services, having activated over 50 pre-qualified sites in less than 8 weeks. This swift method guarantees that tests can begin effectively, backed by FDA/EMA/MDR-ready datasets with centralized oversight.
Navigating regulatory challenges is equally important:
- Compliance with Regulations: All recruitment materials and processes must adhere to local and international regulations to ensure ethical standards are met. bioaccess® ensures that its recruitment strategies adhere to these regulations, facilitating smoother study operations.
- Informed Consent: Clearly communicating the study's purpose, risks, and benefits is essential for facilitating informed decision-making among potential participants.
Additionally, addressing barriers to recruitment is crucial:
- Limited Access to Clinical Trial Sites: bioaccess® actively works to improve participation rates by identifying and addressing challenges faced by patients in remote or underserved areas.
- Financial Concerns: Recognizing that transportation, accommodation, and co-pays can deter potential participants, bioaccess® provides support to alleviate these financial burdens.
- Language and Cultural Barriers: Considering the needs of patients with limited English proficiency or from diverse cultural backgrounds is important for ensuring that information about the study is accessible and comprehensible.
- Diversity in Study Populations: Striving for diverse and representative study populations is essential for the development of innovative treatments and therapies.
By applying these strategies and utilizing bioaccess®'s proficiency in swift patient recruitment and site activation, researchers can improve recruitment efforts, ensure that studies are sufficiently powered, and attain significant outcomes.
Analyze Results: Interpreting Data and Clinical Implications
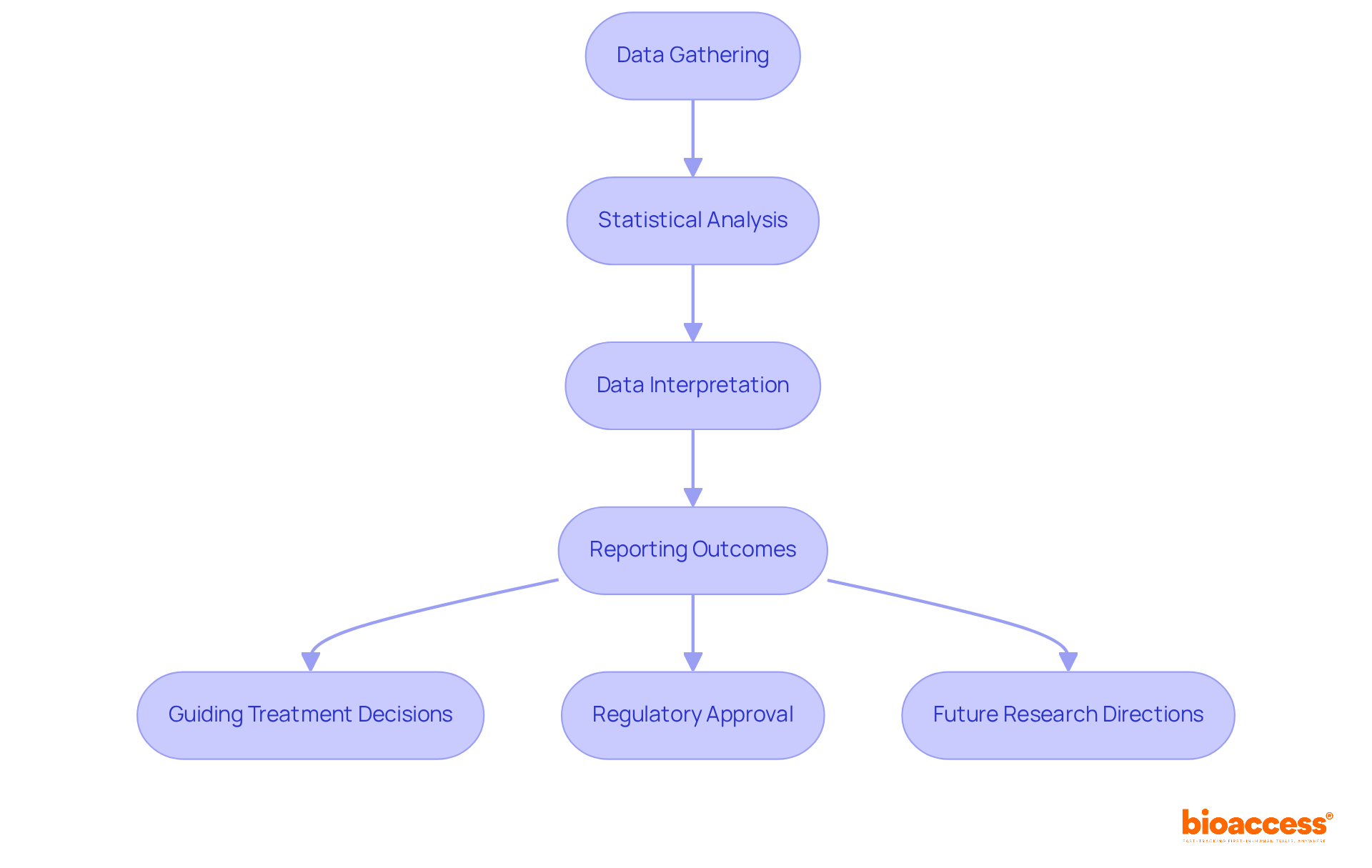
Once data gathering is complete, the next step is to examine the findings, which is a crucial element for the success of phase 3 trials. Key aspects include:
- Statistical Analysis: Utilizing appropriate statistical tests is essential for evaluating both primary and secondary endpoints. Common methods such as t-tests, chi-square tests, and regression analysis are selected based on the data type and research questions.
- Data Interpretation: It is vital to comprehend the significance of the findings in relation to the study aims. This involves assessing clinical significance alongside statistical significance, as the latter alone does not always reflect real-world impact. For instance, a study on advanced pancreatic cancer demonstrated a statistically significant median survival difference of 10 days; however, many oncologists deemed this clinically irrelevant due to associated toxicity and costs.
- Reporting Outcomes: Comprehensive reports detailing methodology, outcomes, and conclusions are indispensable. These documents not only support regulatory submissions but also facilitate publication in scientific journals, ensuring transparency and reproducibility.
The clinical implications of the results can be profound:
- Guiding Treatment Decisions: Positive trial outcomes can lead to significant changes in clinical practice guidelines, influencing how treatments are administered.
- Regulatory Approval: Successful study outcomes can streamline the approval process for new therapies, hastening their accessibility to patients.
- Future Research Directions: Findings may reveal areas for further investigation, including potential new indications for existing treatments or novel therapeutic approaches.
In conclusion, thorough analysis and interpretation of phase 3 trials results are essential for advancing medical knowledge and enhancing patient care.
Conclusion
Mastering Phase 3 trials is essential for the successful development of new therapies, as these trials serve as the final hurdle before a drug can gain regulatory approval. The importance of confirming efficacy, monitoring safety, and conducting comparative analyses cannot be overstated; they ensure that new treatments are both effective and safe for public use. By employing robust study designs, comprehensive recruitment strategies, and thorough data analysis, stakeholders can navigate the complexities of clinical trials and enhance their chances of success.
Key strategies such as the implementation of randomization and blinding, the meticulous calculation of sample sizes, and the engagement of healthcare providers in recruitment efforts are critical components for achieving meaningful results. The emphasis on statistical methodologies and the interpretation of findings underscores the necessity of rigorous analysis in determining the clinical implications of trial outcomes. Collectively, these strategies contribute to the advancement of innovative therapies and the enrichment of medical knowledge.
The successful execution of Phase 3 trials is a multifaceted endeavor that requires careful planning, adherence to regulations, and a commitment to ethical practices. By leveraging best practices in trial design and recruitment, and ensuring a thorough analysis of results, researchers can facilitate the approval of new drugs while enhancing patient care and outcomes. The significance of these trials extends beyond regulatory approval, influencing treatment guidelines and shaping the future of healthcare. Engaging with these strategies is crucial for anyone involved in the clinical research landscape, as they pave the way for groundbreaking advancements in medicine.
Frequently Asked Questions
What are Phase 3 trials?
Phase 3 trials are the final step of clinical evaluation before a medication is submitted for regulatory endorsement. They involve extensive participant groups, ranging from hundreds to thousands, and are designed to confirm the effectiveness of an intervention, monitor side effects, and compare the new intervention with standard or placebo alternatives.
What are the primary objectives of Phase 3 trials?
The primary objectives of Phase 3 trials include confirming efficacy, safety monitoring, and comparative analysis. They aim to establish the treatment's effectiveness across a broader population, identify any adverse effects, and evaluate how the new treatment performs against existing therapies.
How effective are drugs in transitioning from Phase 2 to Phase 3?
Only about 30%-40% of drugs transition successfully from Phase 2 to Phase 3.
What is the significance of safety monitoring in Phase 3 trials?
Safety monitoring is crucial as it involves identifying adverse effects that may not have been evident in earlier phases. Approximately 4%-6% of approved drugs are withdrawn from the market due to safety issues, highlighting the importance of rigorous safety monitoring during Phase 3 trials.
What role does comparative analysis play in Phase 3 trials?
Comparative analysis evaluates how the new treatment performs against existing therapies, which is essential for demonstrating its value in the market.
What services does bioaccess provide for Phase 3 trial management?
Bioaccess provides comprehensive clinical study management services including feasibility analyses, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, which are vital for navigating the complexities of regulatory approval.
What percentage of drugs entering Phase 3 trials receive FDA approval?
Approximately 50%-60% of drugs entering Phase 3 trials ultimately receive FDA approval.
Why are Phase 3 trials considered a cornerstone of clinical research?
Phase 3 trials are essential for ensuring that new therapies are both effective and safe for public use, while also contributing to local economies through job creation and healthcare improvements.
List of Sources
- Define Phase 3 Trials: Importance and Objectives
- Clinical Trial Success Rates: How Many Drugs Make It to Market? (Latest Approval Stats) (https://patentpc.com/blog/clinical-trial-success-rates-how-many-drugs-make-it-to-market-latest-approval-stats)
- Explore Design Methodologies: Statistical Approaches and Trial Structures
- Past, present, and future of Phase 3 vaccine trial design: rethinking statistics for the 21st century (https://academic.oup.com/cei/article/219/1/uxae104/7906440)
- Withdrawn NSCLC Drug: Was Ambitious Phase 3 Trial Design to Blame? (https://oncologynewscentral.com/nsclc/withdrawn-nsclc-drug-was-ambitious-phase-3-trial-design-to-blame)
- Kiniksa Pharmaceuticals Announces Trial Design of Planned Phase 2/3 Clinical Trial of KPL-387 in Recurrent Pericarditis | Kiniksa Pharmaceuticals (https://kiniksa.gcs-web.com/news-releases/news-release-details/kiniksa-pharmaceuticals-announces-trial-design-planned-phase-23)
- Protocol and statistical analysis plan for the phase 3 randomised controlled Treatment of Invasively Ventilated Adults with Early Activity and Mobilisation (TEAM III) trial - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC10692544)
- NeuroSense Receives Positive FDA Feedback on Phase 3 Study Design for PrimeC (https://prnewswire.com/news-releases/neurosense-receives-positive-fda-feedback-on-phase-3-study-design-for-primec-302329028.html)
- Implement Recruitment Strategies: Navigating Regulatory Challenges
- Challenges of Clinical Trial Patient Recruitment | Biodexa Pharmaceuticals (https://biodexapharma.com/patient-resource/challenges-of-clinical-trial-patient-recruitment)
- Analyze Results: Interpreting Data and Clinical Implications
- The Reporting and Interpretation of Randomized Clinical Trials (https://jamanetwork.com/journals/jama/fullarticle/2748772)
- Common pitfalls in statistical analysis: Clinical versus statistical significance - PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC4504060)
- Statistical analysis and significance tests for clinical trial data (https://sciencedirect.com/science/article/pii/S1357303925000787)
- Interpreting Clinical Trial Results (https://sciencedirect.com/science/article/pii/S2949788424000510)






