


The article underscores the key objectives and best practices essential for mastering clinical phase 3 trials, which are pivotal in validating new medical interventions. It highlights the necessity of thorough planning, robust participant recruitment, and strict adherence to regulatory standards to secure successful outcomes. Data indicates that 50-60% of medications in this phase receive FDA approval, underscoring its critical role in drug development. This emphasis on meticulous execution and compliance not only enhances the likelihood of success but also positions stakeholders to navigate the complexities of clinical research effectively.
Clinical phase 3 trials are a cornerstone in the drug development process, meticulously designed to validate the safety and efficacy of new therapies. These trials not only generate critical data for regulatory approval but also play a vital role in enhancing patient care and advancing medical knowledge. However, navigating the complexities of these trials presents significant challenges.
How can researchers ensure successful outcomes while adhering to best practices? This article delves into the key objectives and strategies for mastering clinical phase 3 trials, offering insights that could reshape the future of pharmaceutical research.
Clinical studies, such as the clinical phase 3 trial, are meticulously structured research investigations aimed at assessing the safety and effectiveness of new medications, therapies, or medical devices. They play a crucial role in drug development by generating vital data that underpins regulatory approval, ensuring that new therapies are safe for public use. Typically divided into stages, each research phase, such as the clinical phase 3 trial, has distinct goals and methodologies, enabling scientists to gather essential data regarding the intervention's impact on human participants. The significance of clinical phase 3 trials is profound; they serve as the primary mechanism for validating new medical interventions, ultimately enhancing health outcomes and advancing medical knowledge.
For instance, the initial participant in the LCA10 CRISPR research study received the dosage in March 2020, with dosing continuing until July 2022, underscoring the ongoing commitment to groundbreaking therapies. Moreover, statistical significance of treatment effects was observed as early as month three, representing a remarkable improvement over the industry standard of six months or longer. This expedited validation process underscores the importance of research studies in the medical field, especially in a clinical phase 3 trial, where prompt and effective solutions are essential for patient care.
Additionally, bioaccess® leverages over 20 years of experience in Medtech to ensure enrollment is 50% faster than traditional markets, achieving ethical approvals in just 4-6 weeks. Their comprehensive clinical study management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, reinforce the efficiency and effectiveness of the clinical phase 3 trial facilitated by the organization. This ultimately supports Medtech, Biopharma, and Radiopharma startups in navigating the complexities of clinical research while ensuring regulatory excellence.
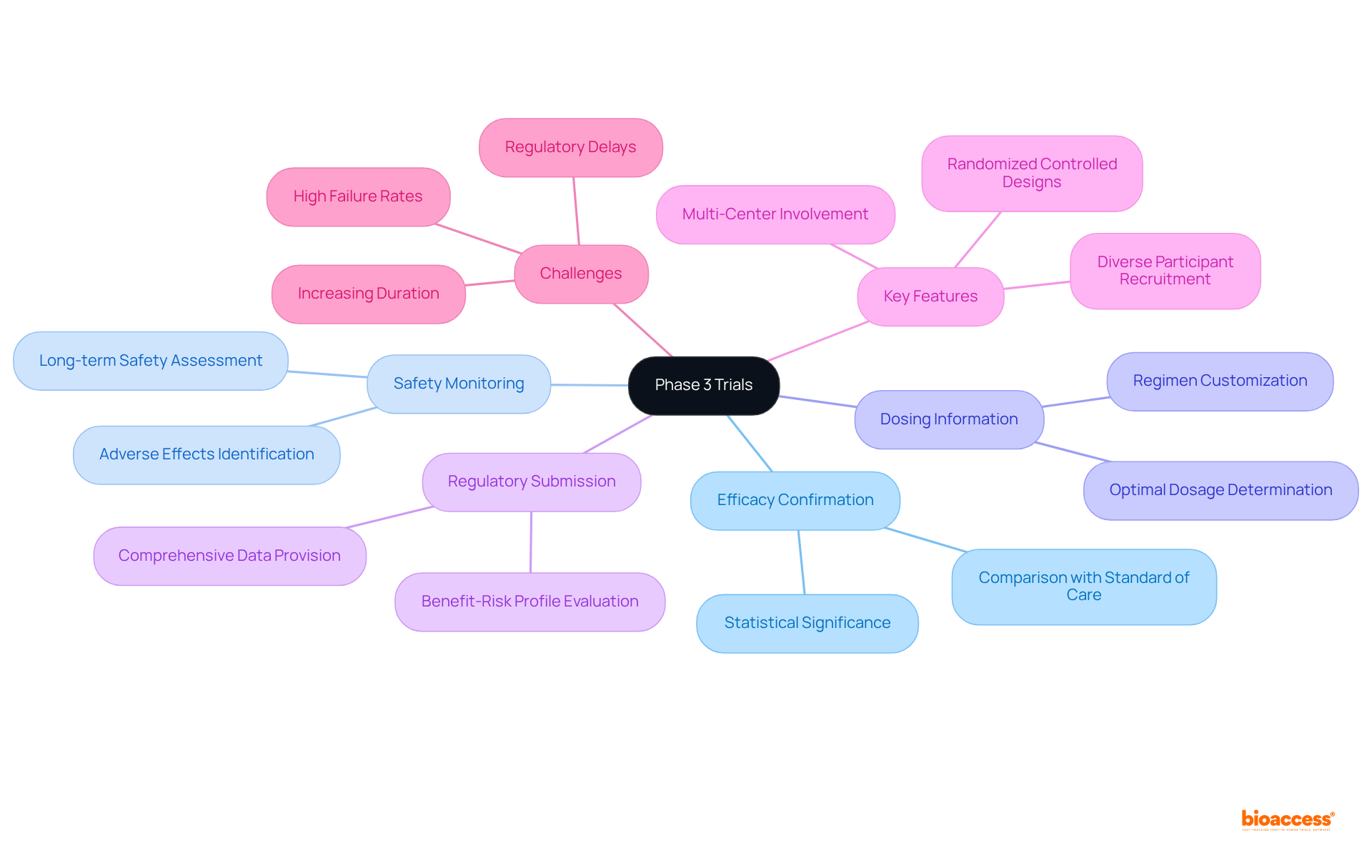
The drug development process relies on clinical phase 3 trials, which are pivotal for verifying the efficacy of a new intervention by comparing it against the existing standard of care. Typically involving large participant groups, these studies enroll between 1,000 and 3,000 individuals, ensuring statistically significant results. The primary objectives of Phase 3 trials include:
Key features of Phase 3 studies include randomized controlled designs, which reduce bias, and multi-center involvement that enhances the diversity and generalizability of outcomes. These assessments are essential for confirming that new treatments are both effective and safe for extensive application. Notably, approximately 50%-60% of medications that reach the clinical phase 3 trial ultimately obtain FDA approval, underscoring the significance of this stage in the clinical research field. Furthermore, the average length of Phase 3 studies has increased to about 3.25 years, reflecting the growing complexity of contemporary research and the necessity for thorough assessment. This duration has risen by about one year over the past decade due to study intricacies and a tight labor market. Additionally, varied participant recruitment is crucial for recognizing safety concerns across populations, ensuring that the findings are relevant to a broader demographic. With bioaccess®'s innovative approach, including a 6-8 week sprint for accelerated patient enrollment, studies can achieve 50% faster enrollment and $25K savings per patient, leveraging FDA-ready data to streamline the process. Moreover, bioaccess® provides extensive clinical study management services, encompassing feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, ensuring a thorough and efficient process for medical device clinical studies.
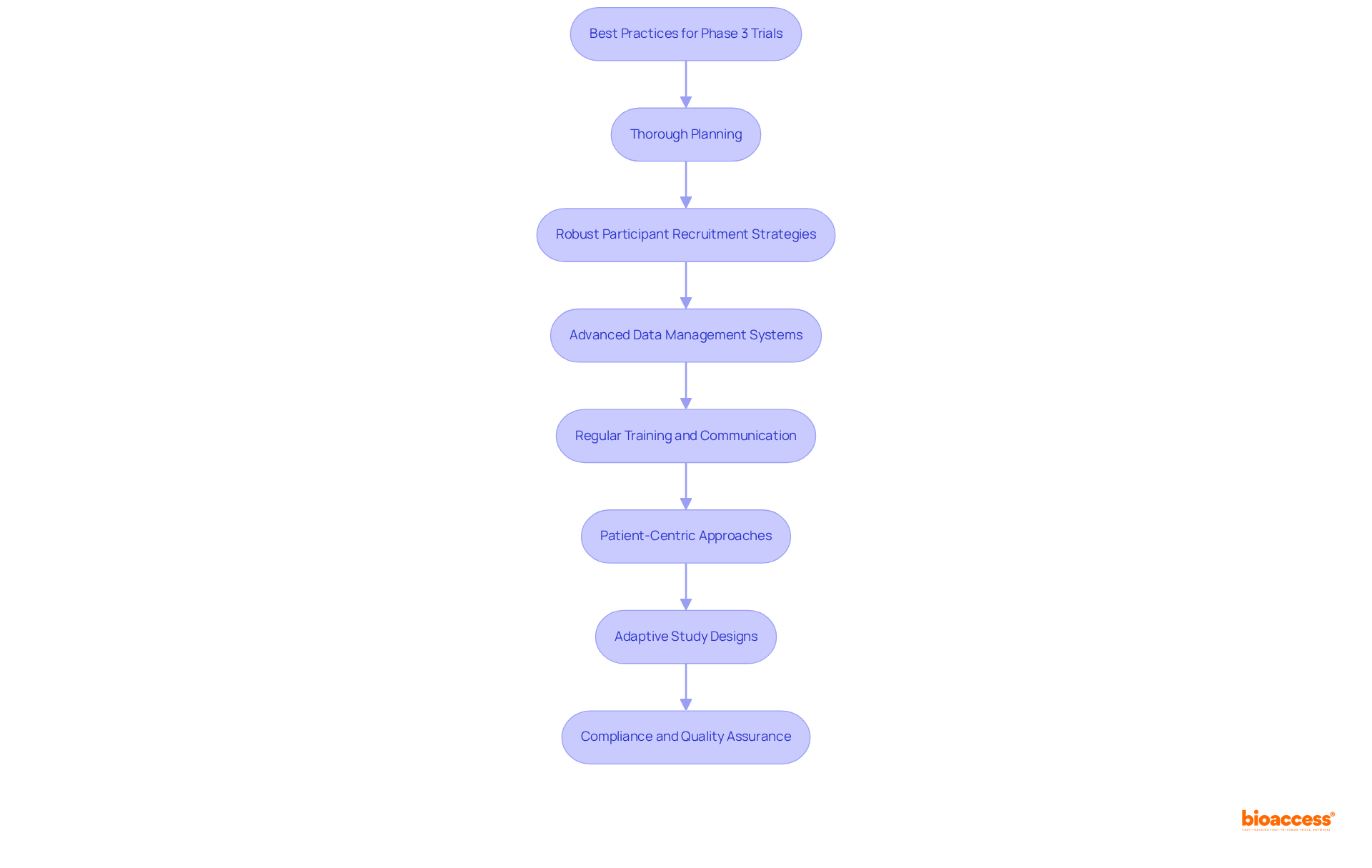
To ensure the success of Phase 3 trials, several best practices must be implemented:
Thorough Planning: Develop a comprehensive trial protocol that clearly outlines objectives, methodologies, and timelines. Engaging stakeholders early fosters alignment of expectations and resource allocation.
Robust Participant Recruitment Strategies: Utilize diverse recruitment channels to reach a broad participant population. Collaborating with healthcare providers and advocacy organizations can significantly enhance outreach initiatives. bioaccess® has demonstrated effectiveness in this area, achieving over a 50% decrease in recruitment time and 95% retention rates through its expedited participant recruitment and site activation services.
Advanced Data Management Systems: Implement advanced data management systems to streamline data collection, monitoring, and analysis. These systems are essential for maintaining data integrity and security, ensuring compliance with regulatory standards.
Regular Training and Communication: Provide ongoing training for research staff while maintaining clear communication channels among all team members. This strategy addresses issues promptly and ensures adherence to protocols.
Patient-Centric Approaches: Incorporate patient feedback into study design and execution. Consider factors such as convenience, accessibility, and support to enhance participant retention and satisfaction.
Adaptive Study Designs: Embrace adaptive study designs that allow for changes based on interim results. This flexibility can improve efficiency and resource distribution, ultimately benefiting the outcomes.
Compliance and Quality Assurance: Establish rigorous quality assurance processes to monitor compliance with regulatory requirements, ensuring the highest standards of research integrity. bioaccess® is dedicated to facilitating regulatory compliance with FDA/EMA/MDR-ready datasets, which is vital for successful multi-region submissions.
By adhering to these best practices, researchers can significantly enhance their chances of achieving successful results in Phase 3 studies, leading to expedited approvals and improved patient access to innovative therapies. Furthermore, collaborations such as that of bioaccess™ and Caribbean Health Group to position Barranquilla as a leading destination for clinical trials in Latin America, supported by Colombia's Minister of Health, exemplify strategic efforts to enhance the clinical research landscape.
Mastering the clinical phase 3 trial is essential for ensuring that new therapies are not only effective but also safe for public use. This stage of drug development serves as a crucial validation point, providing the necessary data for regulatory approval and ultimately enhancing health outcomes. The structured approach to clinical trials, particularly phase 3, underscores the importance of meticulous planning, robust participant recruitment, and adherence to best practices to achieve successful results.
The primary objectives of phase 3 trials include:
With large participant groups and randomized controlled designs, these trials are pivotal in generating statistically significant results that inform regulatory submissions. Insights into best practices—such as thorough planning, advanced data management systems, and patient-centric approaches—demonstrate how researchers can navigate the complexities of clinical trials more effectively, leading to expedited approvals and improved patient access to innovative therapies.
In conclusion, the significance of clinical phase 3 trials cannot be overstated, as they represent a critical juncture in the drug development process. Embracing current best practices and strategic collaborations can enhance the efficiency and effectiveness of these studies, ultimately benefiting public health. As the landscape of clinical research continues to evolve, staying informed about the latest methodologies and objectives will be vital for researchers, stakeholders, and healthcare providers alike, ensuring that new treatments are brought to market safely and effectively.
What are clinical trials and why are they important in drug development?
Clinical trials are structured research investigations designed to assess the safety and effectiveness of new medications, therapies, or medical devices. They are crucial in drug development as they generate essential data that supports regulatory approval, ensuring new therapies are safe for public use.
What is the purpose of clinical phase 3 trials?
Clinical phase 3 trials serve as the primary mechanism for validating new medical interventions. They aim to gather significant data regarding the intervention's impact on human participants, ultimately enhancing health outcomes and advancing medical knowledge.
Can you provide an example of a clinical trial?
An example is the LCA10 CRISPR research study, where the first participant received the dosage in March 2020, with dosing continuing until July 2022. This study demonstrated an expedited validation process, with statistical significance of treatment effects observed as early as month three.
How does bioaccess® contribute to the efficiency of clinical trials?
Bioaccess® leverages over 20 years of experience in Medtech to achieve enrollment that is 50% faster than traditional markets and secures ethical approvals in just 4-6 weeks. They offer comprehensive clinical study management services, enhancing the efficiency and effectiveness of clinical phase 3 trials.
What types of studies does bioaccess® manage?
Bioaccess® manages various types of studies, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, supporting Medtech, Biopharma, and Radiopharma startups in navigating clinical research complexities while ensuring regulatory excellence.