


Phase 3 clinical studies are pivotal for validating the effectiveness and safety of new treatments in comparison to standard therapies. These studies typically involve large patient groups and rigorous methodologies, ensuring that the data collected is reliable for regulatory approval.
However, the article underscores the significant challenges these studies encounter, including:
Furthermore, it highlights the critical importance of ethical standards in clinical research and the essential role of organizations like bioaccess® in streamlining these processes. This collaboration is vital for overcoming obstacles and enhancing the overall efficiency of clinical trials.
Phase 3 clinical studies represent a pivotal stage in medical research, functioning as the critical juncture where new treatments undergo rigorous testing against established therapies to validate their safety and efficacy. These trials possess the potential to transform patient care, yet they encounter a multitude of challenges—from participant recruitment to regulatory compliance—that can impede their success.
What strategies can researchers implement to navigate these obstacles and guarantee that innovative therapies reach those in need? Delving into the complexities of Phase 3 trials uncovers the fragile equilibrium between innovation and ethical responsibility, illuminating the future landscape of drug development.
Phase 3 clinical study evaluations represent pivotal experiments designed to ascertain the effectiveness and safety of new treatments in comparison to standard therapies. These investigations typically encompass extensive patient groups, often ranging from hundreds to thousands of participants, and aim to validate initial findings from earlier phases. The importance of this third stage of testing lies in its ability to provide robust data that regulatory agencies require for medication approval. Such evaluations are instrumental in determining whether a new treatment offers significant advantages over existing options, ultimately shaping clinical practice and enhancing patient outcomes.
For instance, a successful Stage 3 study can lead to the authorization of a new medication that markedly improves patient quality of life or survival rates, underscoring the critical nature of these investigations within the healthcare framework. Additionally, conducting clinical studies in Colombia can yield cost reductions exceeding 30% compared to North America and Western Europe, with a regulatory approval process that typically spans just 4-6 weeks.
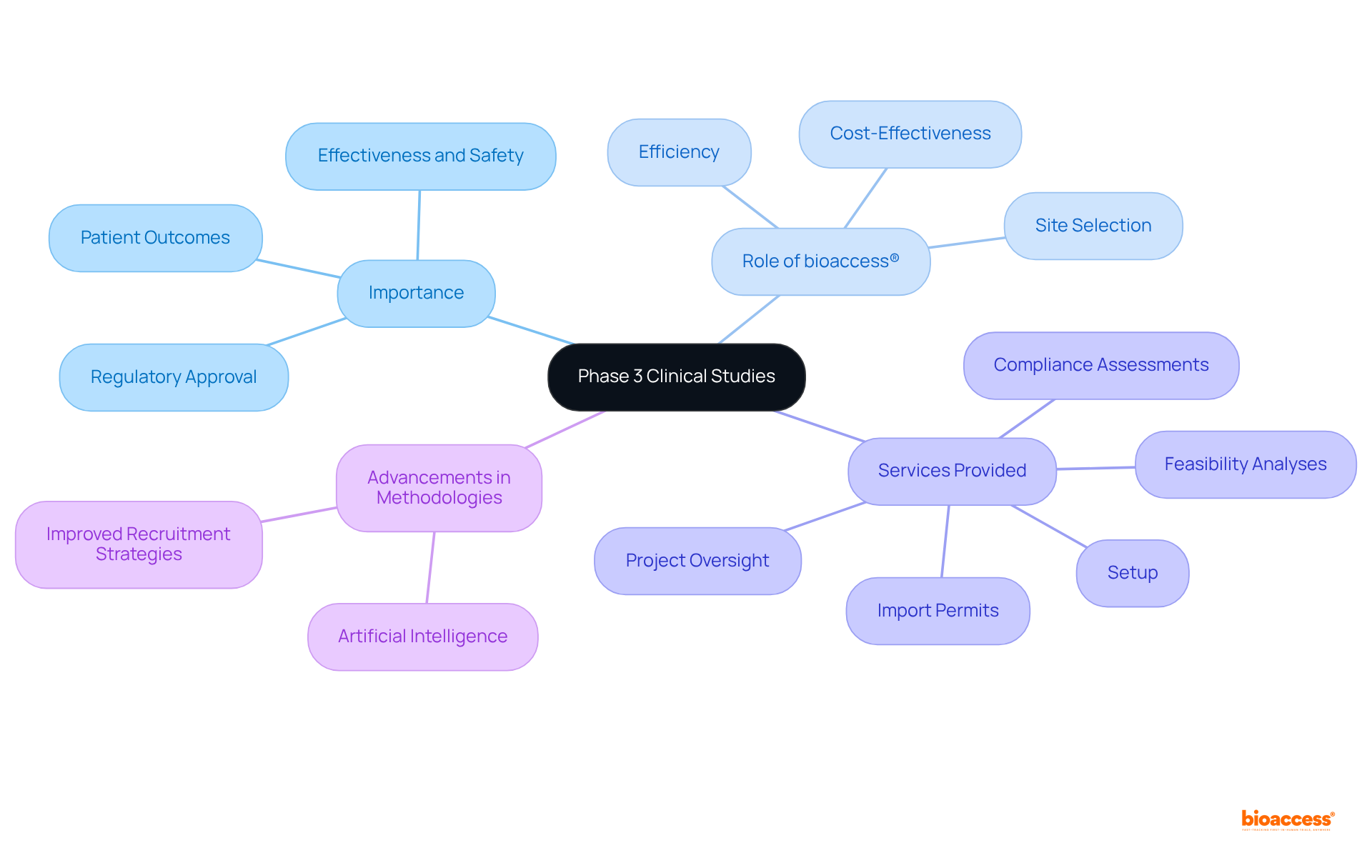
bioaccess® plays an essential role in expediting the phase 3 clinical study by leveraging strategic site selection in Colombia, which enhances both efficiency and cost-effectiveness of the study. Their comprehensive clinical evaluation management services include:
Recent advancements in research methodologies, such as the integration of artificial intelligence and improved subject recruitment strategies, further optimize the efficiency and effectiveness of these studies, highlighting bioaccess's unique contributions to the healthcare landscape.
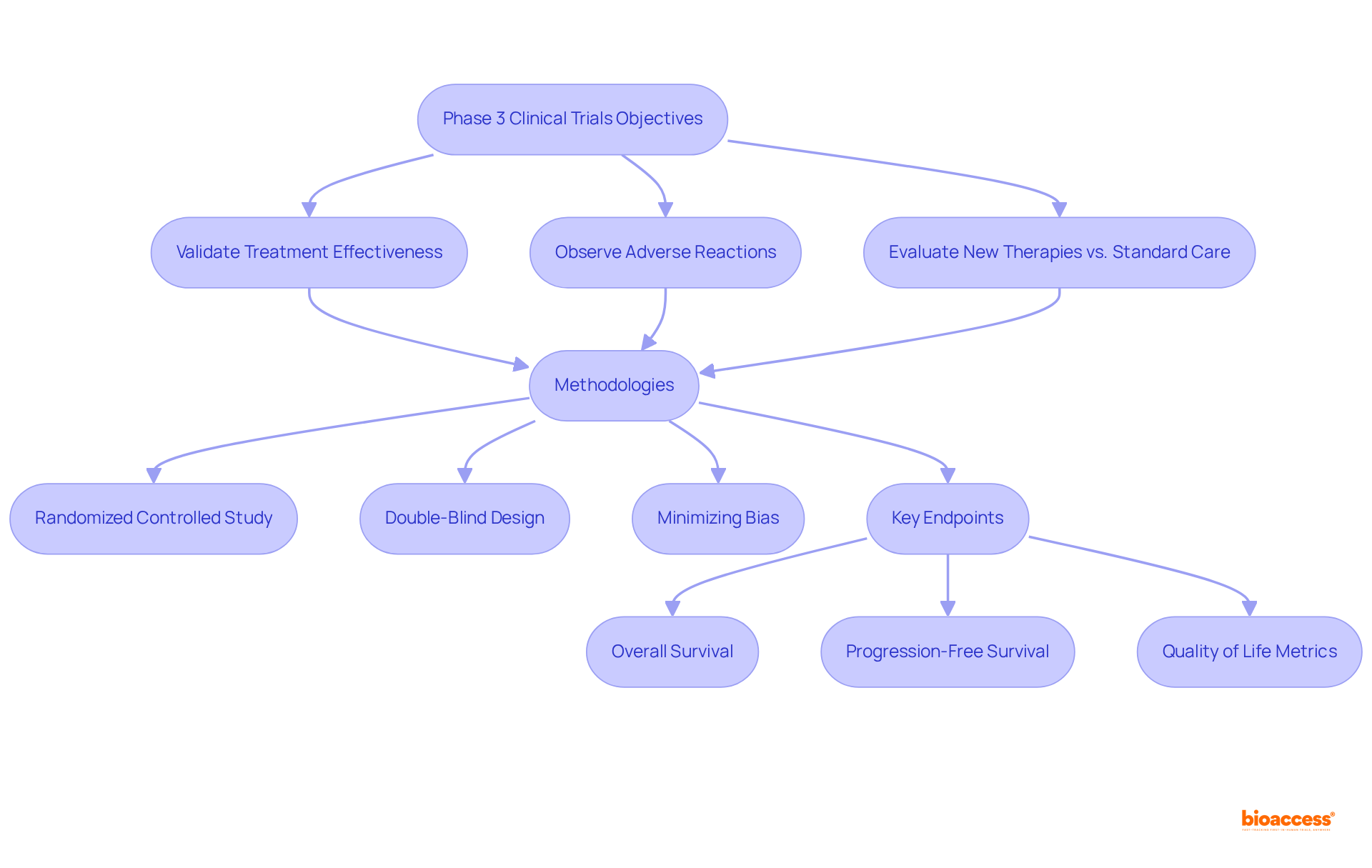
The primary aim of a phase 3 clinical study is to validate treatment effectiveness, observe adverse reactions, and evaluate new therapies in comparison to standard care. These experiments typically employ randomized controlled study methodologies, where participants are randomly assigned to receive either the new treatment or a placebo/control. This randomization minimizes bias, allowing for a clear comparison of outcomes.
Furthermore, many Stage 3 studies utilize a double-blind design, ensuring that neither subjects nor researchers are aware of the treatment assignments, which further reduces bias. Key endpoints assessed in these studies include overall survival, progression-free survival, and quality of life metrics—essential factors for evaluating the treatment's impact on patients.
Data indicate that phase 3 clinical studies often involve several hundred to thousands of individuals and can extend beyond five years, underscoring their complexity and significance in the drug development process. The integration of robust methodologies and comprehensive statistical analysis is crucial for generating reliable data that informs regulatory decisions and clinical practice.
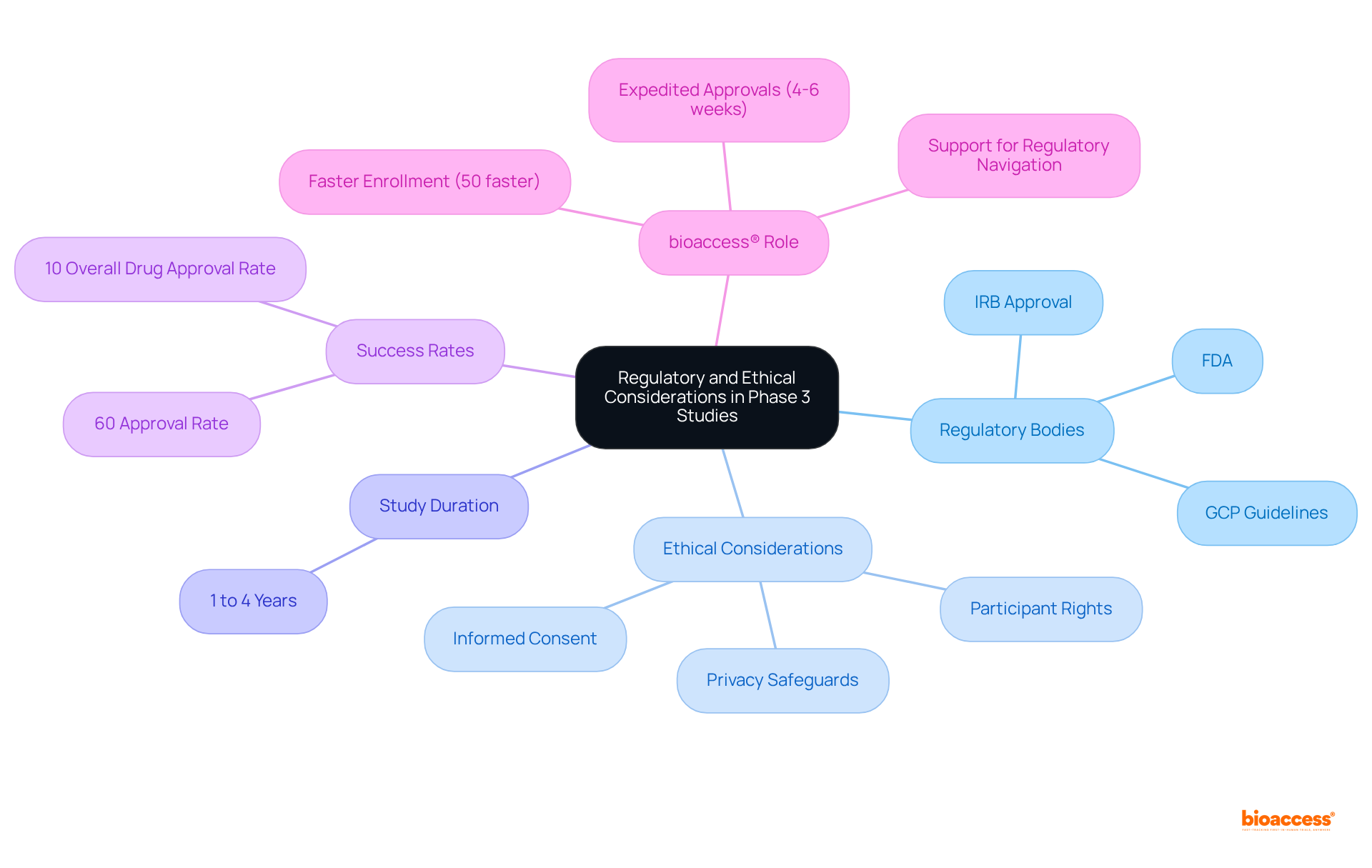
Phase 3 clinical studies are subject to rigorous oversight, ensuring the safety of participants and upholding ethical standards. Regulatory bodies, including the FDA, require comprehensive documentation and strict adherence to Good Clinical Practice (GCP) guidelines. Before commencing a phase 3 clinical study, researchers must obtain approval from an Institutional Review Board (IRB), which assesses the ethical integrity of the study design and the informed consent process.
Ethical considerations are paramount; participants must be fully informed of the potential risks and benefits of the study, and their privacy must be safeguarded. Importantly, individuals retain the right to withdraw from the study at any time without penalty.
These guidelines are vital for protecting participants and preserving the integrity of the research, ultimately contributing to the success of approximately 60% of drugs that advance through this critical phase 3 clinical study and achieve regulatory approval. Phase 3 clinical studies typically require a commitment of 1 to 4 years, underscoring the substantial resources and time invested in this pivotal phase of drug development.
Furthermore, it is essential to recognize that only about 10% of medications that undergo clinical testing ultimately receive FDA approval, illustrating the challenges faced in this field.
bioaccess® enhances the efficiency of this process by delivering ethical approvals in just 4-6 weeks, achieving enrollment 50% faster than traditional methods, and leveraging pre-qualified networks for swift site activation. Additionally, bioaccess® supports innovators with FDA/EMA/MDR-ready datasets and facilitates multi-region submissions, effectively navigating these complexities and accelerating clinical study outcomes.
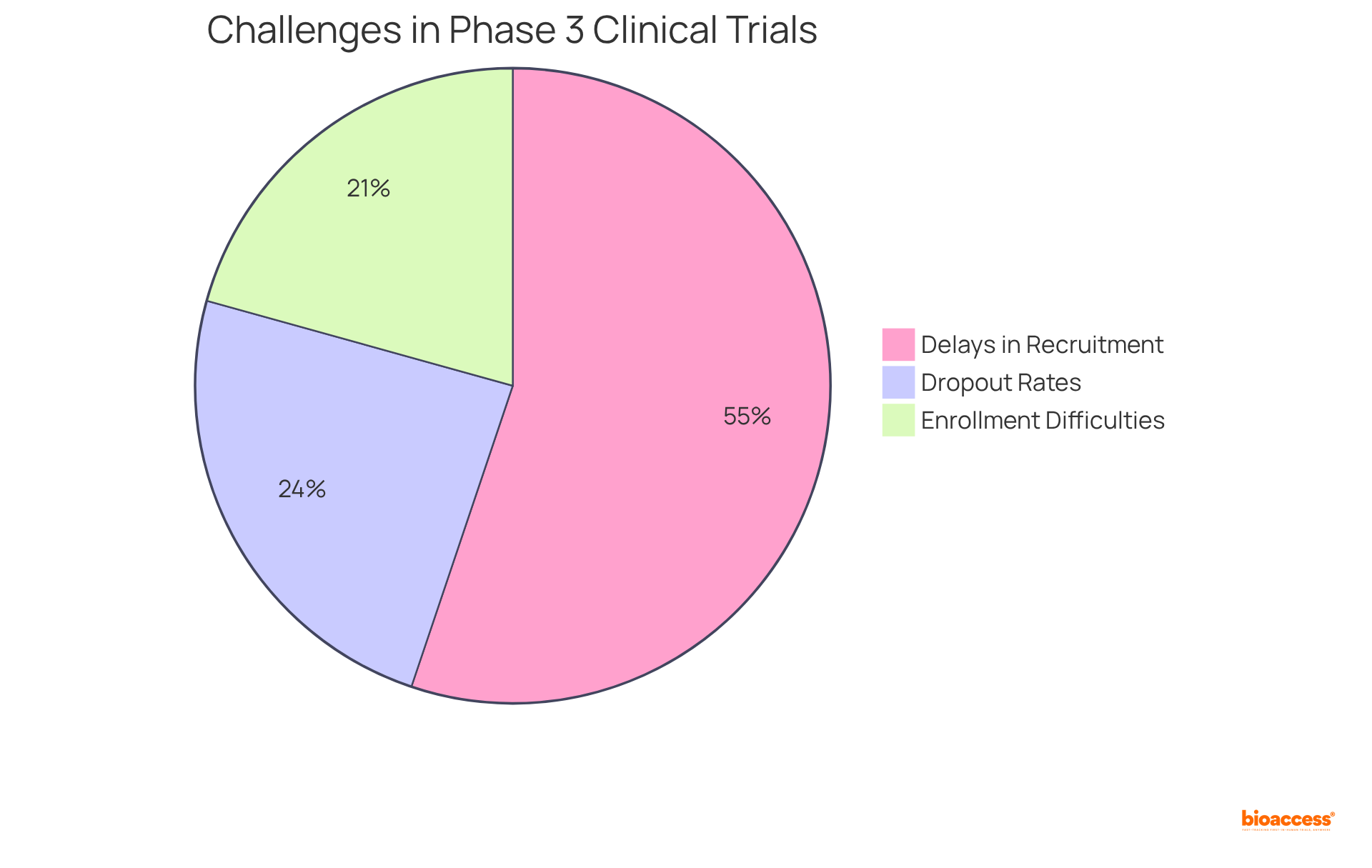
Carrying out a phase 3 clinical study involves navigating a complex array of obstacles, particularly in patient recruitment, retention, and regulatory adherence. A primary challenge is the recruitment of a sufficient number of qualified individuals, often hindered by stringent inclusion and exclusion criteria. Notably, statistics indicate that:
Furthermore, sustaining participant involvement throughout the study is crucial; dropout rates can significantly distort results and undermine the credibility of findings. In fact, over 35% of study participants withdraw due to difficulties in understanding informed consent, underscoring the necessity for clear communication and support.
Regulatory hurdles also present substantial challenges, as researchers must navigate intricate approval processes and comply with evolving guidelines. Additionally, financial constraints complicate the execution of trials, as Stage 3 studies typically require significant investments in resources and personnel.
Proactively addressing these challenges is essential for the successful completion of the phase 3 clinical study, ensuring that innovative therapies reach the market efficiently and effectively.
Phase 3 clinical studies represent a vital link between promising initial research and the eventual approval of new therapies that can revolutionize patient care. These studies not only validate the effectiveness and safety of new treatments but also furnish the comprehensive data essential for regulatory approval. Their pivotal role in shaping clinical practice is undeniable, as they ascertain whether innovative therapies can markedly improve patient outcomes compared to existing options.
This article has delved into the complexities of Phase 3 trials, including their objectives, methodologies, and the stringent regulatory and ethical considerations that govern them. Key insights underscore the challenges encountered in patient recruitment and retention, which significantly influence the success rates of these trials. Furthermore, the contributions of organizations like bioaccess® underscore the necessity of strategic planning and efficient management in surmounting these obstacles, ultimately facilitating the progression of new medications.
Given the challenges and intricacies associated with Phase 3 clinical studies, it is essential for stakeholders in the healthcare sector to prioritize ethical practices, robust methodologies, and effective recruitment strategies. By doing so, the pathway to introducing innovative treatments to the market can be streamlined, ensuring that patients gain timely access to safer, more effective therapies. This unwavering commitment to excellence in clinical research not only enhances the drug development process but also upholds the integrity of the healthcare system as a whole.
What are Phase 3 clinical studies?
Phase 3 clinical studies are pivotal experiments designed to evaluate the effectiveness and safety of new treatments compared to standard therapies, involving extensive patient groups typically ranging from hundreds to thousands of participants.
Why are Phase 3 clinical studies important?
They provide robust data required by regulatory agencies for medication approval and help determine if a new treatment offers significant advantages over existing options, thereby shaping clinical practice and enhancing patient outcomes.
What can a successful Phase 3 study lead to?
A successful Phase 3 study can lead to the authorization of a new medication that significantly improves patient quality of life or survival rates.
How does conducting clinical studies in Colombia benefit the process?
Conducting clinical studies in Colombia can lead to cost reductions exceeding 30% compared to North America and Western Europe, with a regulatory approval process that typically takes just 4-6 weeks.
What role does bioaccess® play in Phase 3 clinical studies?
bioaccess® plays a crucial role in expediting Phase 3 clinical studies by leveraging strategic site selection in Colombia, enhancing both efficiency and cost-effectiveness.
What services does bioaccess® provide for clinical evaluation management?
bioaccess® provides services including feasibility analyses, compliance assessments, setup, import permits, and project oversight.
How have recent advancements in research methodologies impacted Phase 3 studies?
Recent advancements, such as the integration of artificial intelligence and improved subject recruitment strategies, have optimized the efficiency and effectiveness of Phase 3 studies.