


The article emphasizes the mastery of Phase 3 trials in drug development, outlining their critical objectives, inherent challenges, and stringent compliance requirements. These trials are pivotal for confirming a drug's effectiveness and safety. However, they also present challenges such as patient recruitment and regulatory compliance. To navigate these hurdles, advanced methodologies and ethical considerations are essential, ensuring successful market entry for new therapies.
Phase 3 trials represent a pivotal moment in the drug development process, where the potential of a new medication undergoes rigorous examination. These comprehensive studies not only affirm a drug's effectiveness and safety but also act as a crucial gateway for regulatory approval, shaping the future of patient care.
Yet, the complexities inherent in these trials introduce significant challenges, ranging from recruitment difficulties to stringent compliance requirements. What strategies can researchers implement to navigate these obstacles and facilitate the successful launch of groundbreaking therapies?
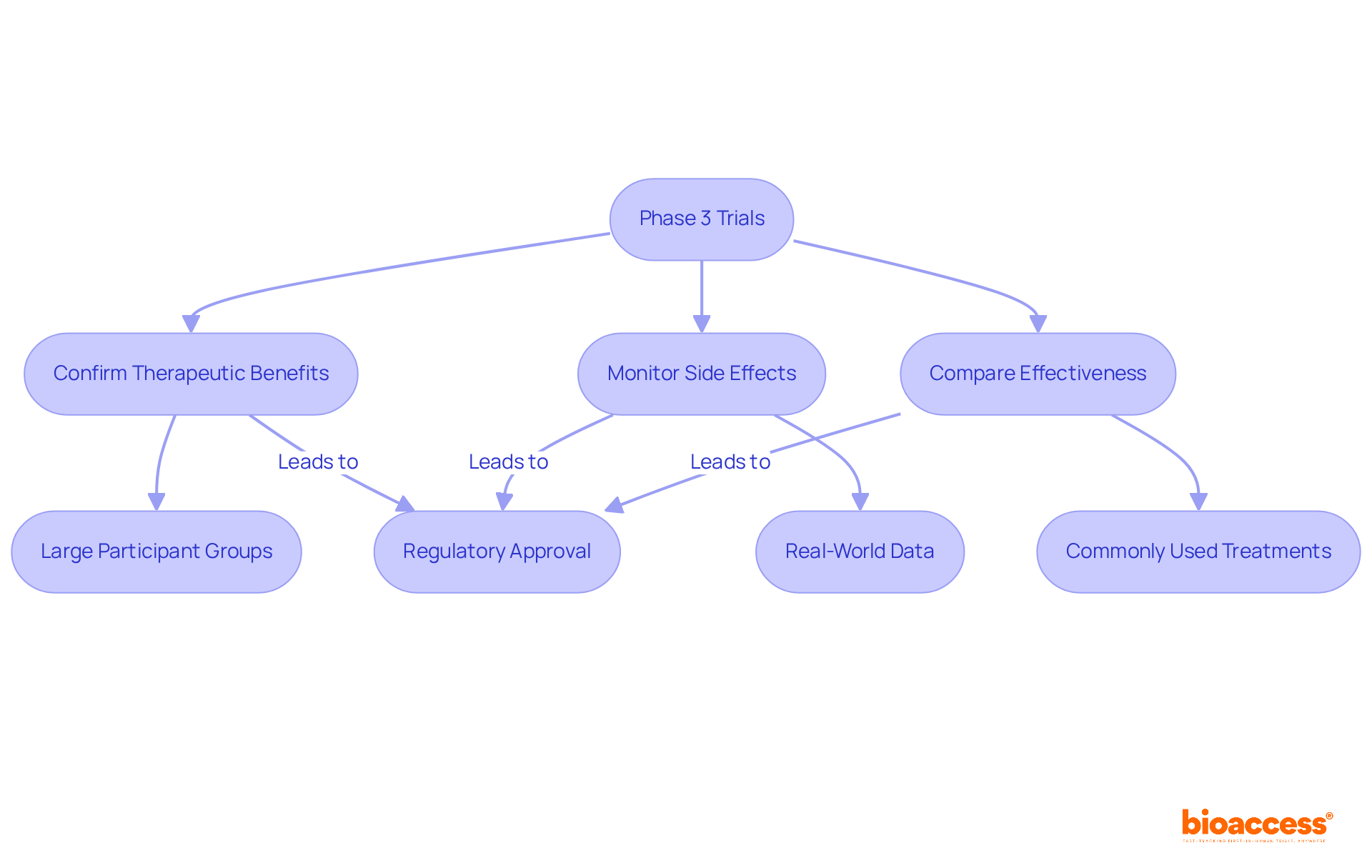
Phase 3 trials represent crucial investigations conducted after initial proof of a medication's effectiveness and safety has been established in prior stages. Typically involving large participant groups, a phase 3 trial aims to confirm the therapeutic benefits of the drug, monitor side effects, and compare its effectiveness against commonly used treatments. The results of the phase 3 trial are vital for regulatory endorsement; approximately 50%-60% of medications that reach this phase 3 trial ultimately obtain FDA authorization.
In 2025, the importance of these evaluations is underscored by their provision of robust data on a drug's performance in real-world settings, particularly following the phase 3 trial, ensuring that new therapies are both effective and safe for widespread use. Successful cases in the Medtech sector illustrate how phase 3 trials can lead to significant advancements in treatment alternatives, reinforcing their role as a pathway to market entry and patient access.
As the drug development landscape evolves, the insights gained from these studies, particularly the phase 3 trial, remain indispensable for regulatory authorities and the pharmaceutical industry alike.
The phase 3 trial is pivotal in drug development, primarily aimed at validating the drug's effectiveness, observing adverse reactions, and comparing the new treatment with existing therapies. With bioaccess®, you can enroll treatment-naive cardiology or neurology groups 50% faster than conventional Western locations, achieving remarkable efficiencies in the process. This acceleration not only enhances enrollment but also results in $25K savings per patient, due to FDA-ready data that eliminates rework and delays.
Common methodologies employed in these studies include:
Statistical analysis is crucial for interpreting the data collected during these experiments. It ensures that findings are not just statistically significant but also clinically relevant. The design often incorporates adaptive methodologies, allowing for adjustments based on interim results, thereby improving the process's efficiency and effectiveness.
Expert insights underscore the importance of rigorous statistical techniques in the phase 3 trial studies. These methodologies are essential for establishing a robust benefit-risk relationship, which is vital for regulatory submissions. Concerns regarding selective reporting and information manipulation in industry-supported studies highlight the necessity for transparency and integrity in clinical research.
The integration of advanced Clinical Trial Management Systems (CTMS) further optimizes participant management and data collection, enhancing compliance and operational efficiency throughout the research process. bioaccess®'s comprehensive clinical study management services encompass:
This ensures that all necessary documentation for regulatory submission is accurate and readily accessible. Overall, the strategies employed in the phase 3 trial are meticulously crafted to ensure that new therapies are both safe and effective before they reach the marketplace.

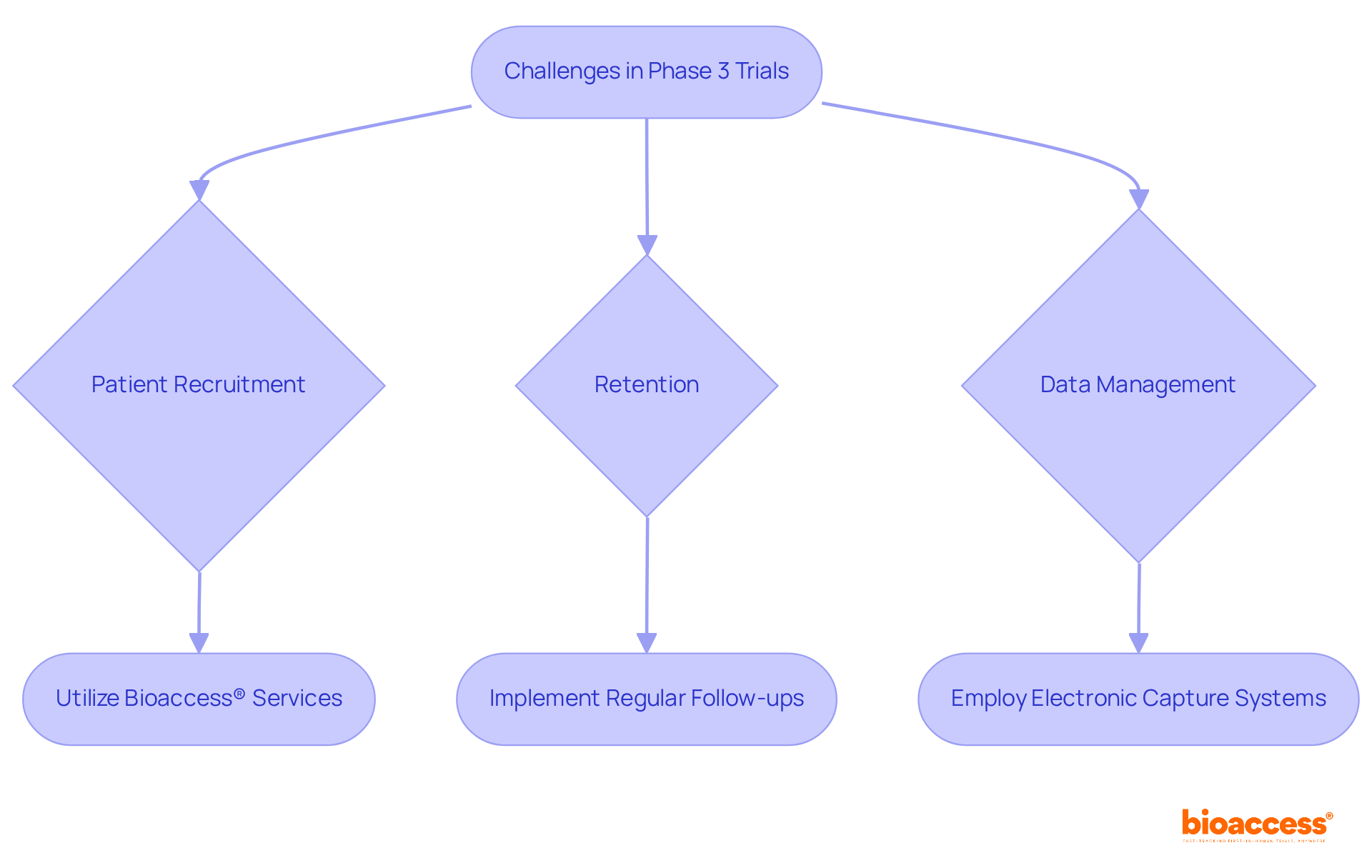
Carrying out a phase 3 trial presents several challenges, particularly in patient recruitment, retention, and data management. Recruitment often suffers due to stringent eligibility criteria and competition from other trials. To effectively tackle this, researchers can utilize bioaccess®'s accelerated patient recruitment services, which facilitate connections between innovative Medtech, Biopharma, and Radiopharma startups and over 50 pre-qualified clinical research sites, all activated in less than eight weeks. This targeted outreach strategy, coupled with collaboration with healthcare providers, significantly enhances the identification of potential candidates.
Retention poses another significant challenge, as participants may withdraw for reasons such as side effects or personal circumstances. Implementing regular follow-ups and offering support can substantially improve retention rates. Furthermore, bioaccess® ensures that all datasets comply with FDA, EMA, and MDR standards, which is crucial for maintaining information integrity. Effective information management is vital, given the substantial amount of data that must be accurately collected and analyzed. By employing electronic information capture systems, this process can be streamlined, reducing errors, and bolstered by bioaccess®'s expertise in delivering reliable study results.
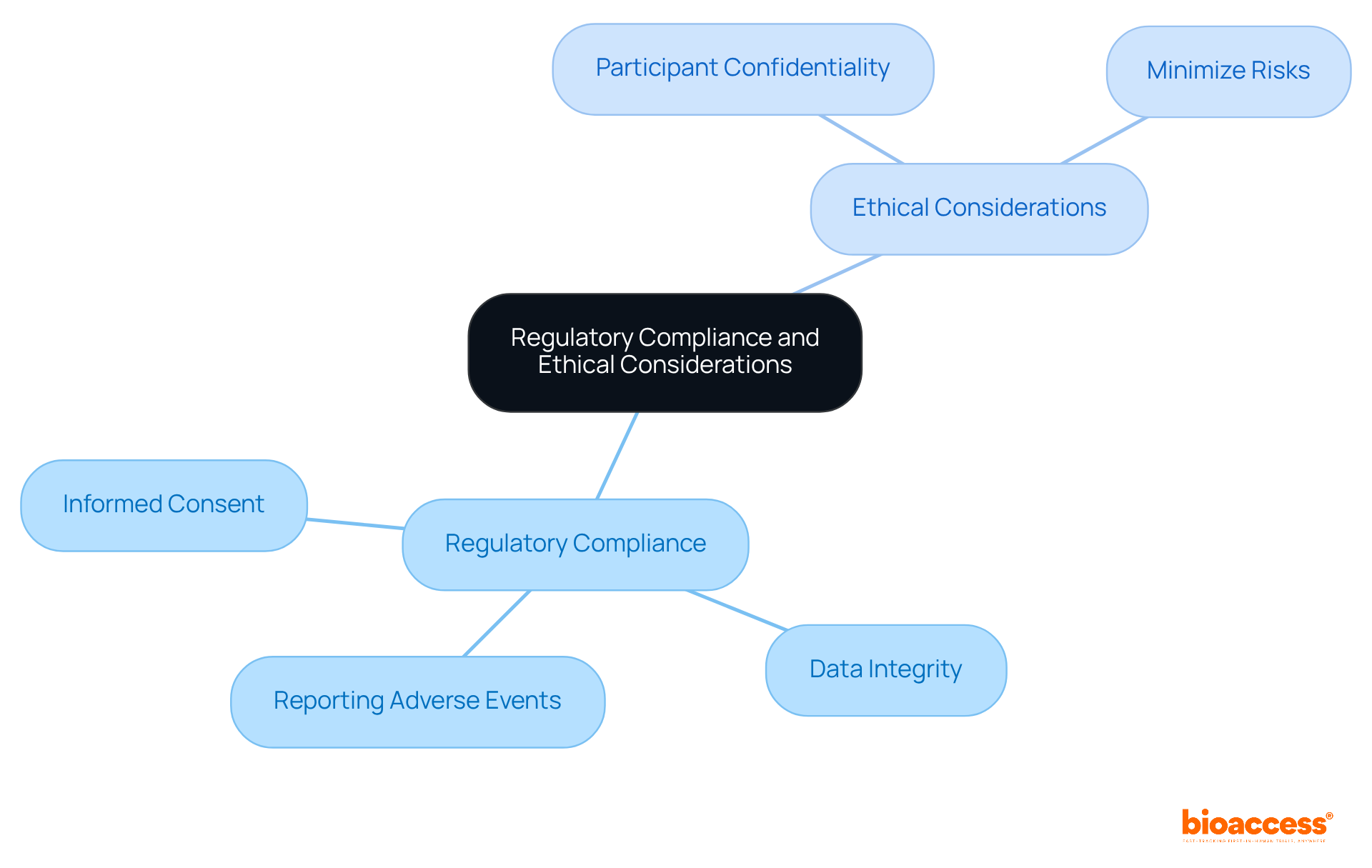
Regulatory compliance and ethical considerations are paramount in the phase 3 trial, particularly for organizations like bioaccess®, a leading contract research organization (CRO) specializing in medical device clinical trials in Latin America. Researchers must adhere to guidelines established by regulatory agencies such as the FDA or EMA, which necessitate:
At bioaccess®, our expertise in regulatory navigation guarantees that these guidelines are meticulously followed, thereby enhancing the credibility of our studies. Ethical considerations further encompass:
Institutional Review Boards (IRBs) play a critical role in reviewing research protocols to ensure that ethical standards are met. By prioritizing compliance and ethics, bioaccess® fosters trust and credibility in its studies, ultimately contributing to the advancement of medical knowledge. Katherine, our Regulatory Affairs Specialist, exemplifies this commitment through her extensive experience in guiding medical device manufacturers through the regulatory landscape, ensuring that all trials meet the highest ethical standards.
Phase 3 trials are crucial in drug development, representing the final assessment to validate a medication's safety and effectiveness prior to regulatory approval. These trials confirm therapeutic benefits, monitor side effects, and compare new treatments with existing options, ensuring that only safe and effective therapies reach the market.
The article highlighted essential aspects of phase 3 trials, including their objectives, methodologies, and challenges. Methodologies such as randomized controlled trials and robust statistical analyses are vital for securing reliable results. Challenges like patient recruitment and data management were addressed, underscoring innovative solutions that enhance the trial process. Furthermore, the significance of ethical standards and regulatory compliance was emphasized as fundamental to maintaining the integrity of clinical research.
In conclusion, phase 3 trials play a pivotal role in shaping the future of medical treatments. The insights derived from these trials are indispensable for regulatory authorities and the healthcare community. By embracing best practices and innovative strategies, the industry can enhance patient safety and foster trust in new medical advancements, ultimately leading to improved healthcare outcomes for patients.
What are Phase 3 trials in drug development?
Phase 3 trials are crucial investigations conducted after initial proof of a medication's effectiveness and safety has been established in earlier stages. They typically involve large participant groups and aim to confirm the drug's therapeutic benefits, monitor side effects, and compare its effectiveness against commonly used treatments.
Why are Phase 3 trial results important?
The results of Phase 3 trials are vital for regulatory endorsement, as approximately 50%-60% of medications that reach this phase ultimately obtain FDA authorization. They provide robust data on a drug's performance in real-world settings, ensuring new therapies are effective and safe for widespread use.
How do Phase 3 trials impact the Medtech sector?
Successful cases in the Medtech sector demonstrate that Phase 3 trials can lead to significant advancements in treatment alternatives, reinforcing their role as a pathway to market entry and patient access.
What role do Phase 3 trials play in the evolving drug development landscape?
As the drug development landscape evolves, the insights gained from Phase 3 trials remain indispensable for regulatory authorities and the pharmaceutical industry, ensuring the continued assessment of drug effectiveness and safety.