


The role of the principal investigator (PI) in clinical trials is vital for overseeing the study's design, implementation, and adherence to regulatory and ethical standards, thereby ensuring participant safety and data integrity. PIs must exhibit strong leadership, communication, and problem-solving skills to effectively navigate challenges, including regulatory hurdles and participant recruitment. This expertise is essential for facilitating successful research outcomes, reinforcing the importance of their position within the clinical research landscape.
The role of the principal investigator (PI) in clinical trials is pivotal, serving as the cornerstone for the successful execution of research that can lead to groundbreaking medical advancements. Understanding the intricacies of this position not only illuminates the responsibilities involved but also underscores the critical skills and qualifications necessary for effective leadership in clinical settings.
However, the journey to mastering this role is fraught with challenges, ranging from navigating complex regulatory landscapes to ensuring participant safety and maintaining data integrity.
What strategies can aspiring principal investigators employ to surmount these obstacles and excel in their vital roles?
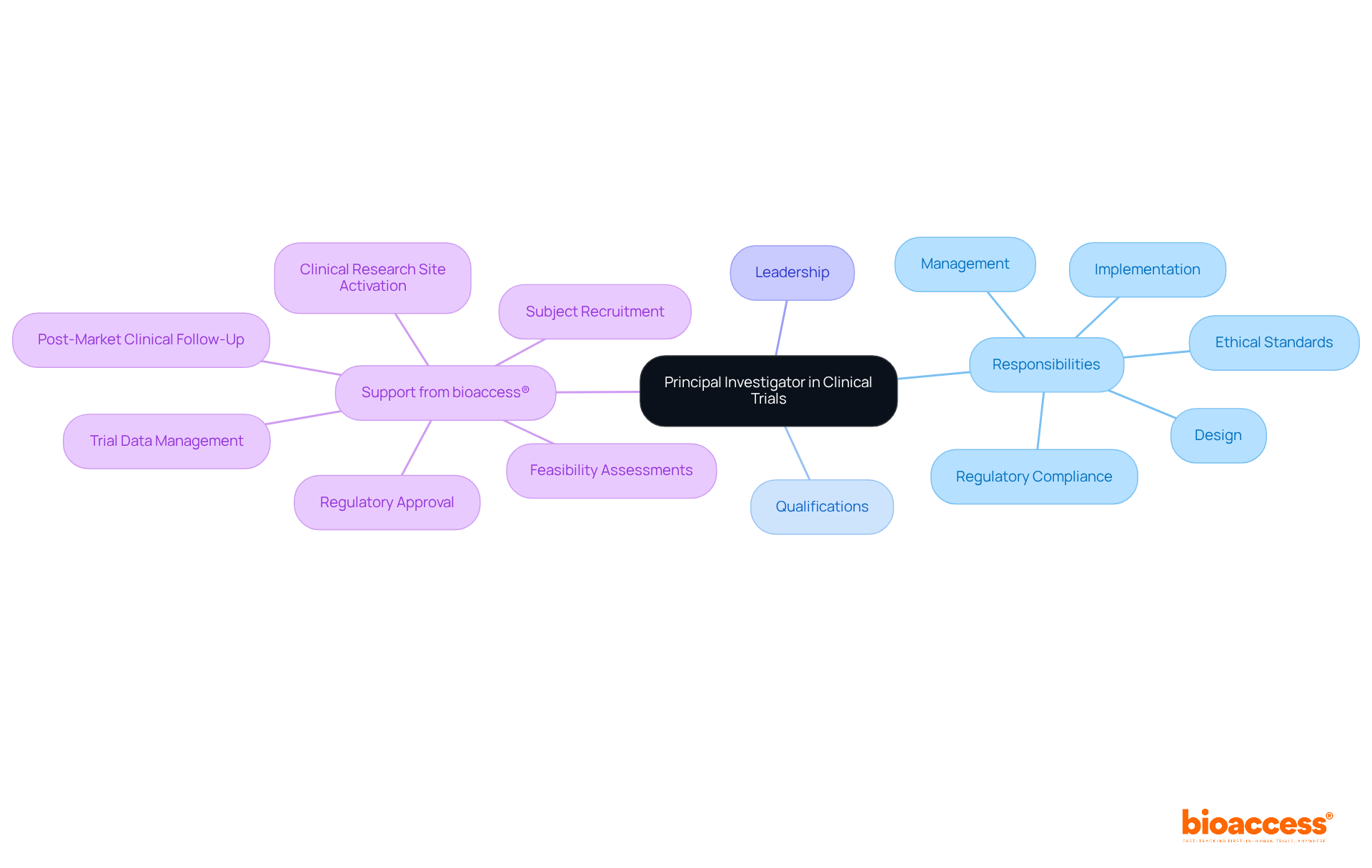
The individual responsible for the overall conduct of a clinical trial, particularly in the context of medical devices, is referred to as the principal investigator clinical trial. This role encompasses the design, implementation, and management of the study, ensuring compliance with regulatory requirements and ethical standards.
At bioaccess®, a leading contract development organization (CRO) in Latin America, we acknowledge the critical importance of this role. The principal investigator clinical trial, typically a certified medical expert or scientist, directs the clinical study team, making pivotal decisions about the study's trajectory while safeguarding participant safety and data integrity. Their leadership is essential for fostering collaboration among team members and adhering to Good Clinical Practice (GCP) guidelines.
With bioaccess®'s expertise in accelerating clinical research outcomes—including regulatory approval, clinical research site activation, subject recruitment, and trial data management—PIs can rely on tailored support that enhances their ability to navigate the complexities of clinical trials, from early feasibility assessments (EFS) to pivotal trials and post-market clinical follow-up (PMCF).
The key responsibilities of a Principal Investigator encompass several critical areas:
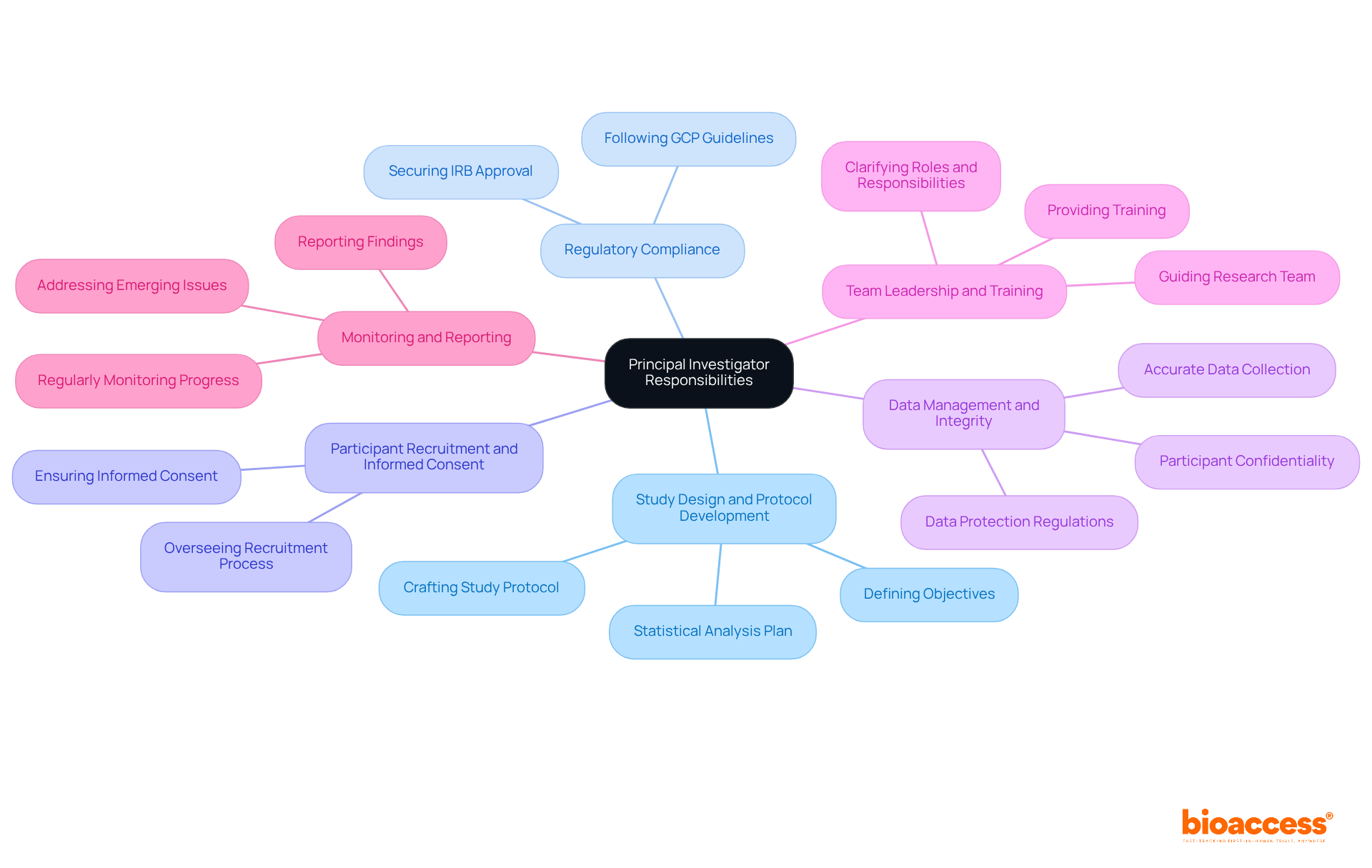
To become a successful Principal Investigator, individuals typically need the following qualifications:

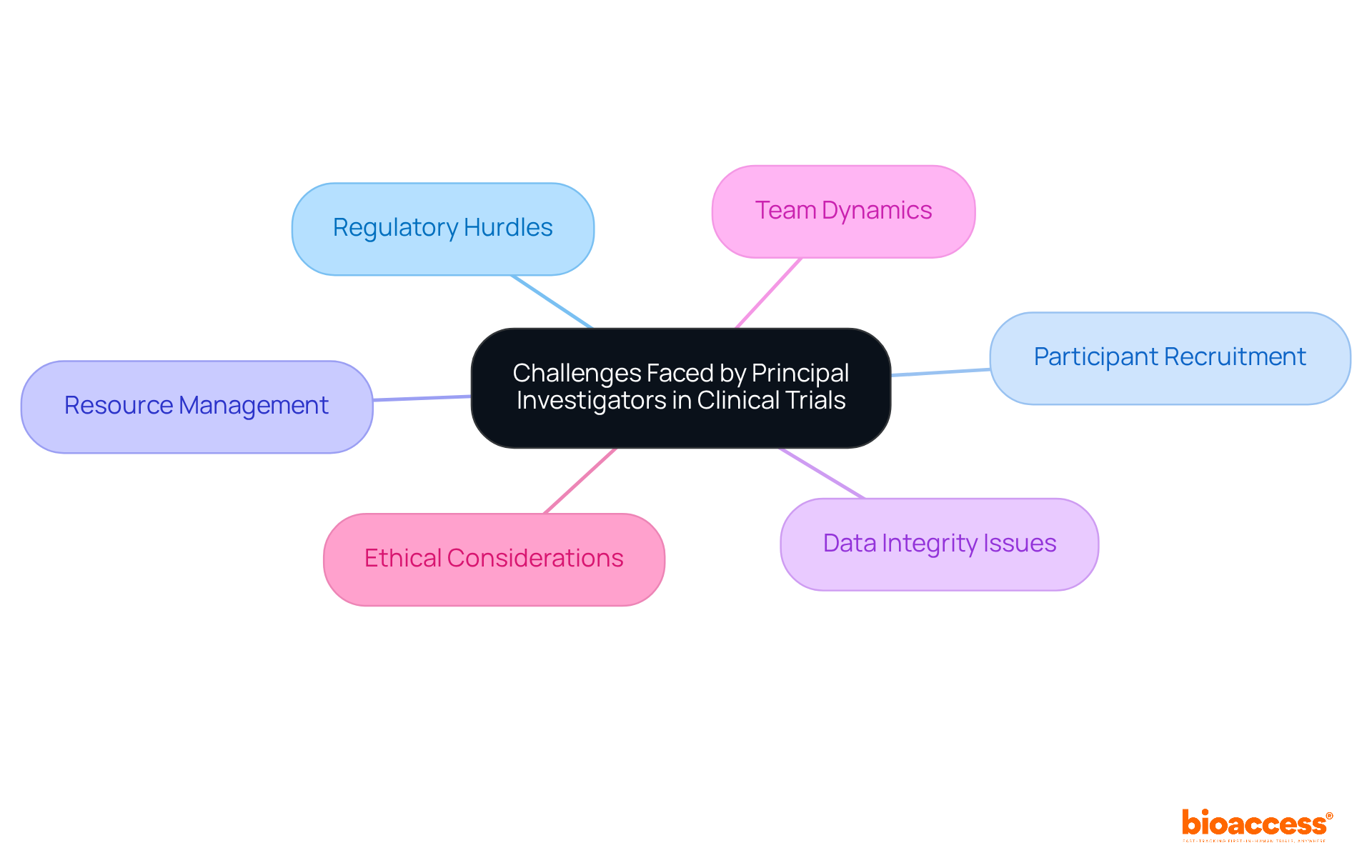
The principal investigator clinical trial encounters several significant challenges, which are critical to understand for successful research outcomes. These challenges include:
The role of the principal investigator in clinical trials is pivotal to the success of research endeavors, particularly in the realm of medical devices. This individual not only oversees the entire study but also ensures adherence to regulatory standards and ethical guidelines, ultimately safeguarding participant safety and data integrity. Understanding the multifaceted responsibilities of a principal investigator is crucial for anyone involved in clinical research, as this role is instrumental in guiding the trial from inception to conclusion.
Throughout the article, key responsibilities of the principal investigator were outlined, including:
Each of these areas is essential for maintaining the integrity of the clinical trial process. Moreover, the qualifications and skills required for a successful principal investigator were also discussed, emphasizing the necessity of a solid educational background, clinical experience, and strong leadership and communication abilities. The challenges faced by principal investigators, such as regulatory hurdles and participant recruitment difficulties, highlight the complexities involved in managing clinical trials.
In light of these insights, it is clear that mastering the principal investigator role is not merely about overseeing a study; it is about fostering collaboration, ensuring compliance, and navigating challenges effectively. As clinical trials continue to evolve, the importance of a well-prepared and resilient principal investigator cannot be overstated. Embracing best practices and seeking continuous support can empower principal investigators to lead their teams successfully, ultimately contributing to advancements in medical research and patient care.
What is the role of a principal investigator in clinical trials?
The principal investigator is responsible for the overall conduct of a clinical trial, including the design, implementation, and management of the study, while ensuring compliance with regulatory requirements and ethical standards.
What qualifications does a principal investigator typically have?
A principal investigator is usually a certified medical expert or scientist.
What are the key responsibilities of a principal investigator?
Key responsibilities include directing the clinical study team, making important decisions about the study's direction, safeguarding participant safety, and ensuring data integrity.
How does the principal investigator contribute to team collaboration?
The principal investigator fosters collaboration among team members, ensuring that everyone adheres to Good Clinical Practice (GCP) guidelines.
What support does bioaccess® provide to principal investigators?
Bioaccess® offers tailored support that enhances principal investigators' ability to navigate the complexities of clinical trials, including assistance with regulatory approval, clinical research site activation, subject recruitment, and trial data management.
What types of clinical trials can a principal investigator oversee?
A principal investigator can oversee various types of clinical trials, from early feasibility assessments (EFS) to pivotal trials and post-market clinical follow-up (PMCF).