


The article underscores the critical importance of defining study needs, evaluating consulting capabilities, and implementing effective communication strategies to maximize success with clinical research consulting services. It highlights the necessity of:
This approach is essential for navigating the complexities of clinical trials efficiently, ensuring that all parties are aligned and informed throughout the process.
Navigating the complex landscape of clinical research can be daunting; however, the right consulting services can transform potential challenges into opportunities for success. By leveraging expert guidance, organizations can precisely define their clinical research needs, ensuring alignment with regulatory requirements and study objectives.
But how can one effectively evaluate the capabilities of consulting firms to ensure they are equipped to deliver on these critical needs? This article delves into best practices for maximizing the success of clinical research through strategic consulting partnerships, offering insights into the essential factors that drive effective collaboration and superior study outcomes.
To enhance the success of clinical study advisory services, it is crucial to begin with a thorough definition of your study requirements. This process involves clearly identifying the specific objectives of your study, the target population, and the desired outcomes. Employing tools such as needs assessments and stakeholder interviews can yield valuable insights. For instance, if your aim is to evaluate a new medical device, delineating success parameters, including safety, efficacy, and patient satisfaction metrics, is essential. This clarity aids in selecting appropriate methodologies and clinical research consulting services, ensuring alignment with your study objectives.
Furthermore, understanding the regulatory landscape and specific compliance requirements that may influence your study is vital. Collaborating with a consultant early in this stage, especially through clinical research consulting services, provides essential insights into these elements, facilitating the enhancement of your goals and simplifying the investigation process. Bioaccess® offers extensive trial management services, including:
These services are crucial for navigating the intricacies of medical studies.
As emphasized by industry leaders, effective stakeholder involvement serves as a cornerstone of successful clinical studies, fostering a more nuanced understanding of participant needs and expectations.

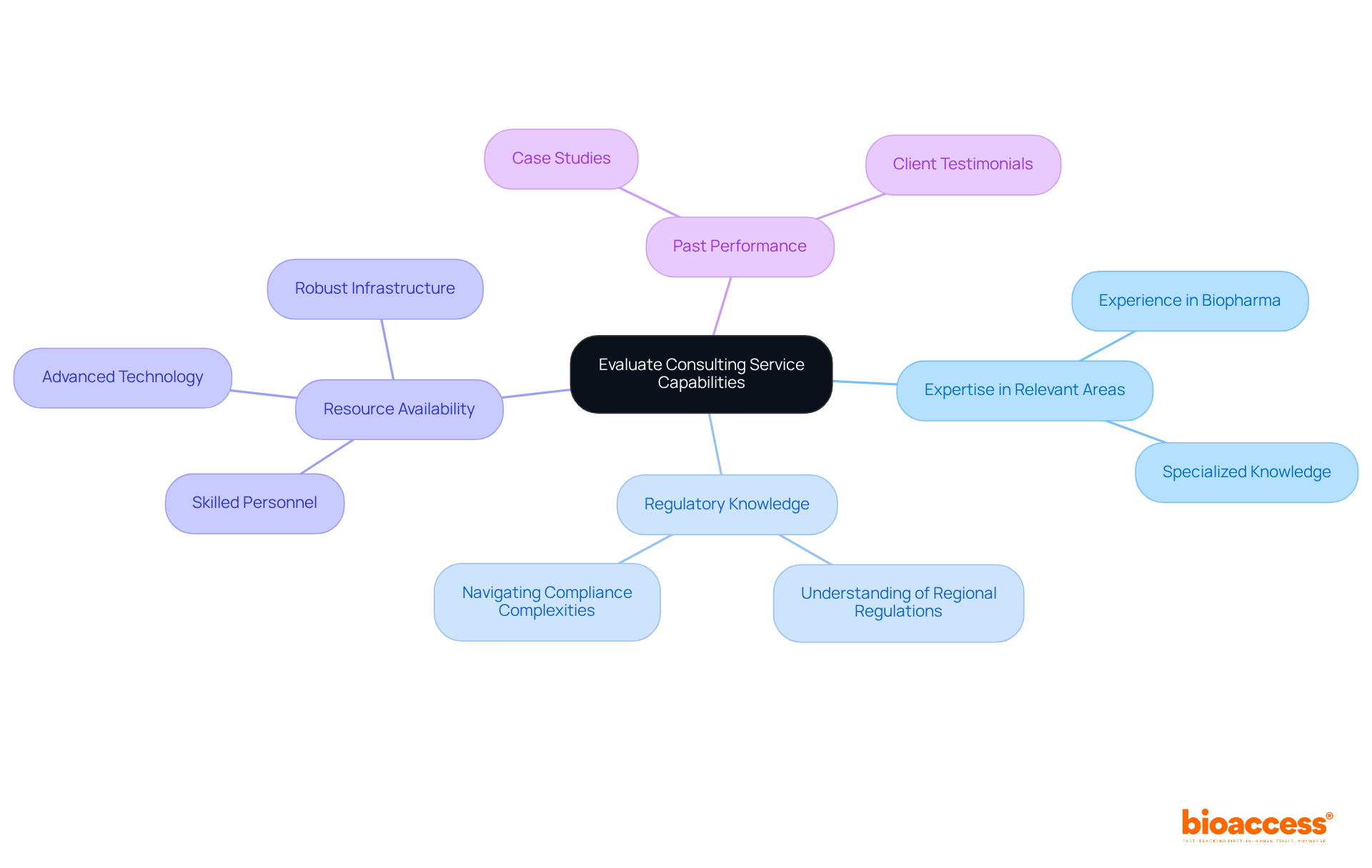
When assessing the capabilities of consulting services, several key factors must be prioritized:
Expertise in Relevant Areas: Ensure that the advisory firm possesses substantial experience in your specific therapeutic area or type of research. For instance, if your study involves a biopharmaceutical product, seek consultants with a proven track record in biopharma; their specialized knowledge can significantly influence study outcomes.
Regulatory Knowledge: A deep understanding of regulatory requirements in your target markets is crucial. This is especially pertinent for studies that span multiple regions, such as Latin America and Australia, where regulatory frameworks can vary widely. Firms excelling in navigating these complexities can expedite the approval process and enhance compliance.
Resource Availability: Assess whether the advisory firm possesses the necessary resources, including skilled personnel, advanced technology, and robust infrastructure, to effectively support your study. Access to diverse patient populations, efficient data management systems, and sophisticated analytical tools is vital for successful trial execution.
Past Performance: Reviewing case studies or testimonials from previous clients provides valuable insights into the firm's success in similar projects. This assessment reveals their ability to deliver results on time and within budget, which is critical for maintaining project timelines and financial efficiency.
By concentrating on these elements, organizations can make educated choices when choosing a partnership, ultimately enhancing the success of their medical investigation initiatives.
To ensure the success of your medical investigation, it is essential to evaluate the experience and skills of potential advisory partners in clinical research consulting services. Here are effective strategies for evaluation:
Review Credentials: Seek consultants with relevant academic qualifications and certifications in research, such as degrees in life sciences or public health, along with recognized certifications. This foundation is crucial, as studies indicate that candidates with formal training are more competitive in the job market and can command higher starting salaries.
Evaluate Industry Experience: Consider the number of years the firm has been operational. Firms with extensive experience in clinical research consulting services are likely to have navigated various challenges, which provides them with valuable insights that can enhance your project's success. For instance, prosperous consulting companies typically possess an operational history exceeding ten years, which correlates with elevated success rates in research studies.
Check for Specialized Knowledge: Depending on your area of study, ensure that the consultants possess specialized knowledge in fields such as regulatory compliance, data management, or patient recruitment strategies. Proficiency in these areas is essential, particularly when around 80% of research studies encounter delays due to recruitment challenges, which emphasizes the need for clinical research consulting services.
Conduct Interviews: Engage potential consultants in discussions to gauge their understanding of your specific needs and their problem-solving approach. This interaction can reveal their depth of knowledge and communication skills, which are vital for effective collaboration. Successful partnerships often stem from clear communication and a shared vision for the project.

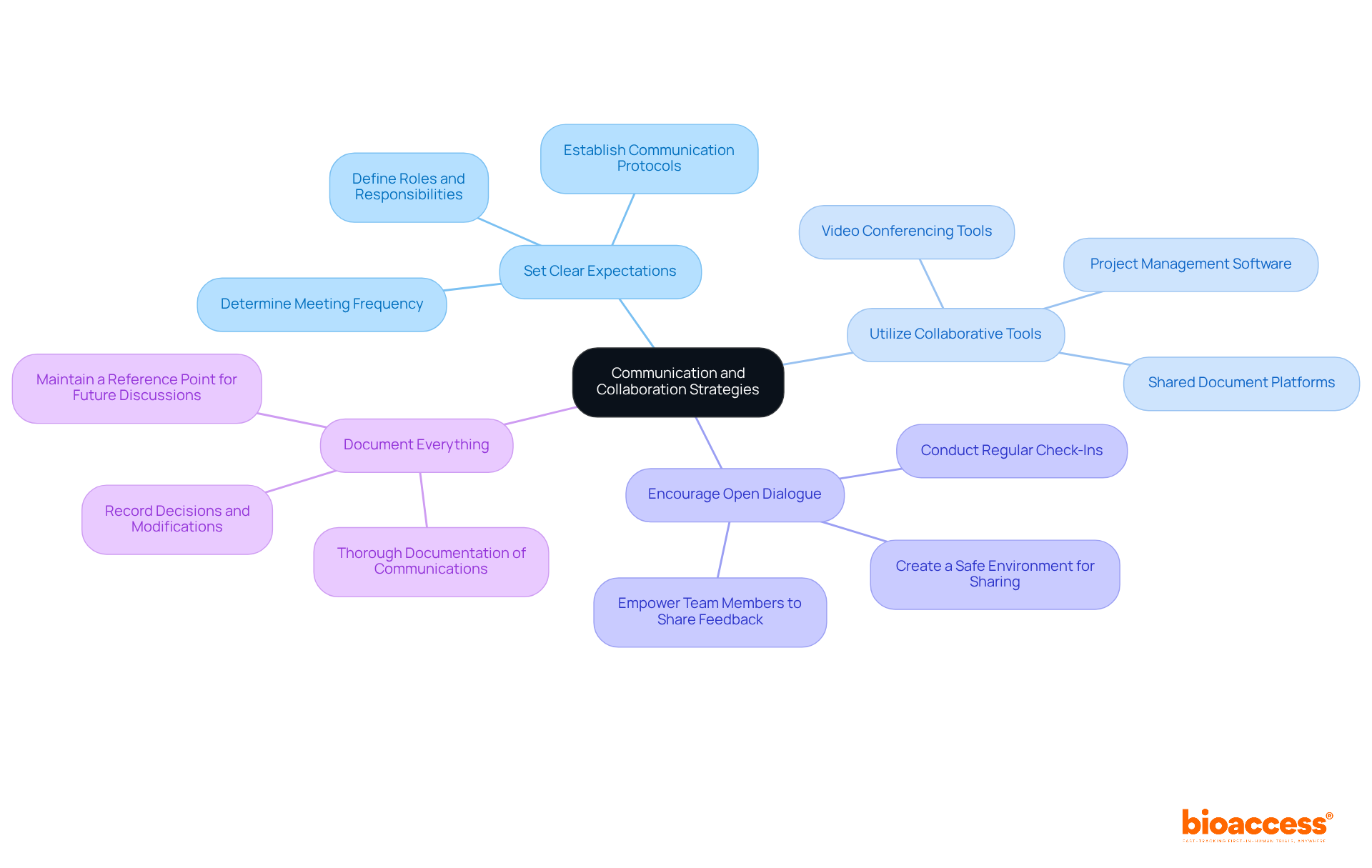
Efficient communication and teamwork are critical components of successful clinical research consulting services, especially within the comprehensive management services offered by bioaccess®. To establish these strategies effectively, consider the following best practices:
Set Clear Expectations: At the project's outset, it is essential to define roles, responsibilities, and communication protocols. This includes determining the frequency of meetings, preferred communication methods, and reporting frameworks, particularly when overseeing complex studies such as Early-Feasibility and First-In-Human research.
Utilize Collaborative Tools: Embrace technology to enhance communication and collaboration. Tools like project management software, shared document platforms, and video conferencing can significantly improve real-time collaboration and keep all stakeholders informed. Research shows that collaboration has surged by at least 50% over the last decade, highlighting the increasing significance of clinical research consulting services in the industry, especially for managing the complexities of clinical trials.
Encourage Open Dialogue: Create an environment where team members feel empowered to share ideas, concerns, and feedback. Regular check-ins can help identify potential issues early and allow for timely adjustments to the study plan. As Mike Krzyzewski aptly noted, "Effective teamwork begins and ends with communication," emphasizing the necessity of open dialogue in collaboration, particularly when navigating regulatory requirements and compliance reviews.
Document Everything: Ensure thorough documentation of all communications, decisions, and modifications to the study plan. This practice not only guarantees accountability but also serves as a reference point for future discussions and evaluations, which is vital in the context of clinical research consulting services and project management.
The impact of collaboration tools on research outcomes is profound. Organizations that champion collaborative work are five times more likely to achieve high performance. As Helen Keller insightfully stated, "Alone, we can do so little; together we can do so much." This sentiment reinforces the transformative potential of teamwork in clinical trials, where effective collaboration can yield innovative solutions, enhance patient outcomes, and contribute significantly to local economies through job creation and healthcare improvement.
Maximizing the potential of clinical research necessitates a strategic approach that encompasses the definition of specific study needs, evaluation of consulting service capabilities, and the fostering of effective communication and collaboration. By meticulously outlining objectives and desired outcomes, organizations can align their research efforts with the appropriate consulting services, ensuring a more streamlined and successful investigation process.
Key insights from the article underscore the importance of selecting consultants with relevant expertise and a proven track record, alongside the necessity of establishing clear communication channels from the outset. Emphasizing stakeholder involvement and utilizing collaborative tools can significantly enhance the research experience, ultimately leading to improved patient outcomes and overall project success.
As the landscape of clinical research continues to evolve, embracing these best practices becomes increasingly vital. Organizations are urged to prioritize effective partnerships and open dialogue within their teams. By doing so, they not only enhance the efficiency of their studies but also contribute to the advancement of medical knowledge and innovation in healthcare.
What is the first step in enhancing the success of clinical study advisory services?
The first step is to thoroughly define your study requirements, including specific objectives, target population, and desired outcomes.
How can one gain valuable insights into their clinical research needs?
Valuable insights can be gained through needs assessments and stakeholder interviews.
Why is it important to delineate success parameters in a clinical study?
Delineating success parameters, such as safety, efficacy, and patient satisfaction metrics, is essential for selecting appropriate methodologies and ensuring alignment with study objectives.
What role does understanding the regulatory landscape play in clinical research?
Understanding the regulatory landscape and specific compliance requirements is vital as it may influence the study and its outcomes.
How can collaborating with a consultant benefit the clinical research process?
Collaborating with a consultant early in the study can provide essential insights into regulatory and compliance elements, enhancing goals and simplifying the investigation process.
What trial management services does Bioaccess® offer?
Bioaccess® offers services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
Why is stakeholder involvement important in clinical studies?
Effective stakeholder involvement is crucial as it fosters a nuanced understanding of participant needs and expectations, which is a cornerstone of successful clinical studies.