


The article examines the key similarities and differences between medical device Good Manufacturing Practices (GMP) in Mexico and global standards. It highlights the regulatory framework established by COFEPRIS and its alignment with ISO 13485. Specific requirements such as sanitary registration and GMP certification are detailed, contrasting them with international practices. This emphasizes the need for local compliance and outlines the implications for Medtech companies seeking market access in Mexico.
Understanding the landscape of Good Manufacturing Practices (GMP) for medical devices in Mexico is crucial for manufacturers aiming to navigate this complex regulatory environment. Recent updates from COFEPRIS enhance product safety and efficacy while streamlining market access for both local and international companies. However, as these regulations align with global standards like ISO 13485, significant differences remain that could pose challenges for compliance. Medtech companies must consider how to effectively adapt to these evolving requirements while ensuring successful entry into the Mexican market.
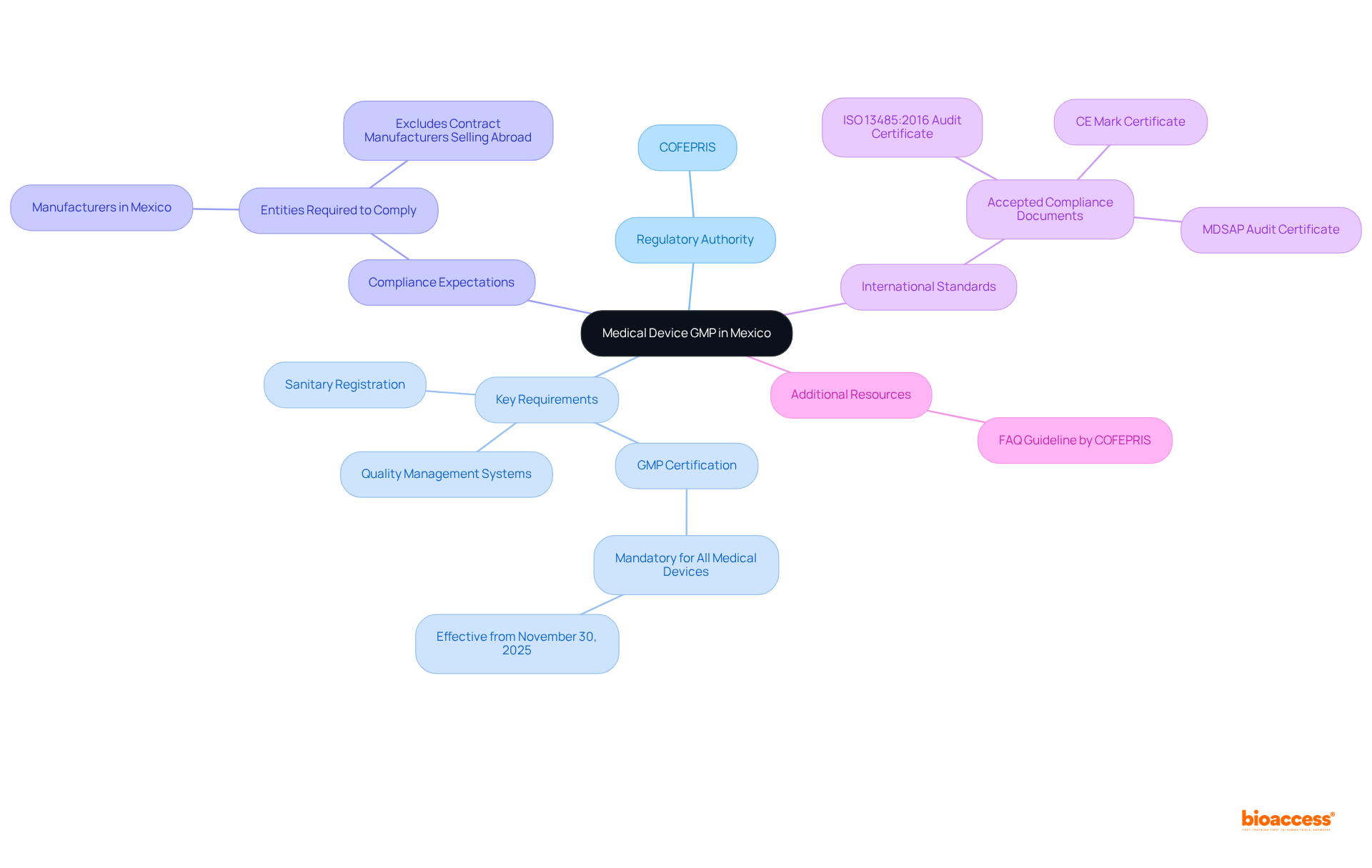
Good Manufacturing Practices (GMP) in the country are regulated by the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS), which has recently updated its guidelines through NOM-241-SSA1-2025. This new standard provides a medical device GMP Mexico overview, establishing a robust framework for the manufacturing of medical devices and ensuring that products are consistently produced and controlled in accordance with stringent quality standards. The medical device GMP Mexico overview includes key requirements such as:
The revised regulations clarify compliance expectations, particularly emphasizing that only entities manufacturing, conditioning, storing, or distributing medical devices for commercialization in Mexico must comply, explicitly excluding contract manufacturers whose products are sold abroad. This strategic approach not only enhances patient safety and product efficacy but also streamlines market access for manufacturers. Significantly, the regulatory authority now acknowledges various internationally accepted documents, such as MDSAP Audit Certificates and CE Mark Certificates, as part of the medical device GMP Mexico overview to expedite compliance and approval processes.
Additionally, COFEPRIS has published an accompanying FAQ guideline to address common inquiries related to the new standard. These updates aim to reduce complexity and improve transparency for stakeholders in the medical device sector, aligning with bioaccess®'s commitment to supporting Medtech, Biopharma, and Radiopharma innovators in navigating these regulatory changes.
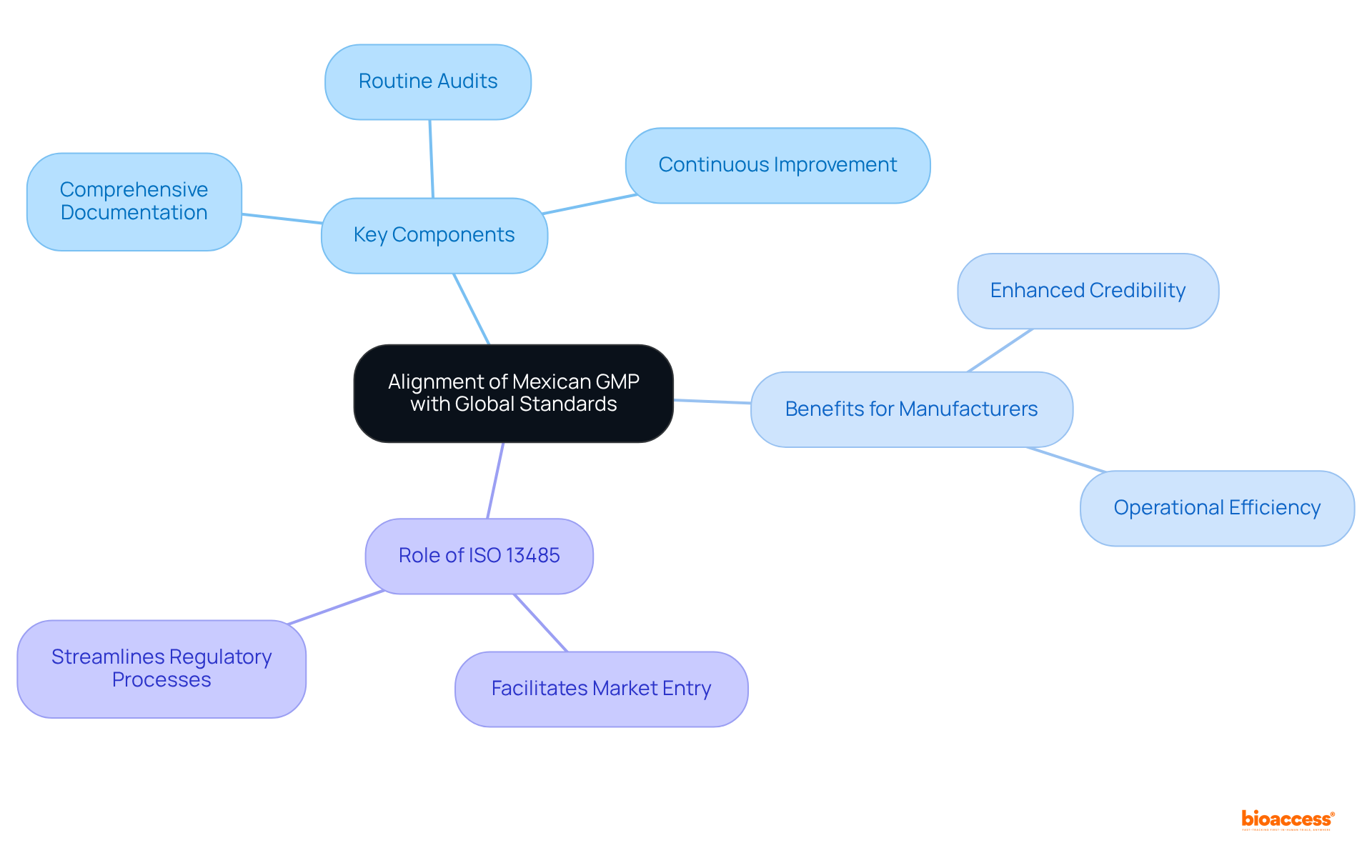
The overview of medical device GMP in Mexico shows significant alignment with global standards, particularly ISO 13485, which is a cornerstone in the medical device sector. This alignment underscores the necessity of a robust quality management system (QMS) to ensure consistent product quality and safety. Key components such as:
are mandated by both frameworks. Notably, the acceptance of ISO 13485 certification as a valid indication of GMP compliance in Mexico is an important aspect of the medical device GMP Mexico overview, facilitating smoother entry for manufacturers already certified and enhancing their operational efficiency. This synergy streamlines regulatory processes and fosters a culture of quality, which is essential for the successful commercialization of the medical device GMP Mexico overview. Companies that align their practices with these standards can significantly enhance their credibility and competitiveness in both local and international markets.
The medical device GMP Mexico overview shows that while it aligns with many global standards, notable differences persist. The health authority imposes specific requirements for sanitary registration that may not be found in other nations. Moreover, timelines for compliance and processes for obtaining GMP certification can vary significantly.
In Mexico, the acceptance of GMP certifications from other countries, as outlined in the medical device GMP Mexico overview, relies on the issuing authority's acknowledgment by COFEPRIS, a condition that may not be applicable in other jurisdictions. Furthermore, the Mexican regulatory framework emphasizes local manufacturing, which can significantly impact foreign companies aiming to enter the market.
Ethical approvals in Mexico can be delivered within 4-6 weeks, offering a relevant comparison to compliance timelines. The anticipated evaluation period for health equipment registration in 2025 is expected to range from 3 to 8 months, underscoring the structured six-step assessment process mandated by COFEPRIS. This focus on local production is particularly significant, considering that nearly 90% of healthcare instruments sold in Mexico are imports.
Thus, understanding these regulatory nuances is essential for successfully navigating the medical device GMP Mexico overview in the industry.
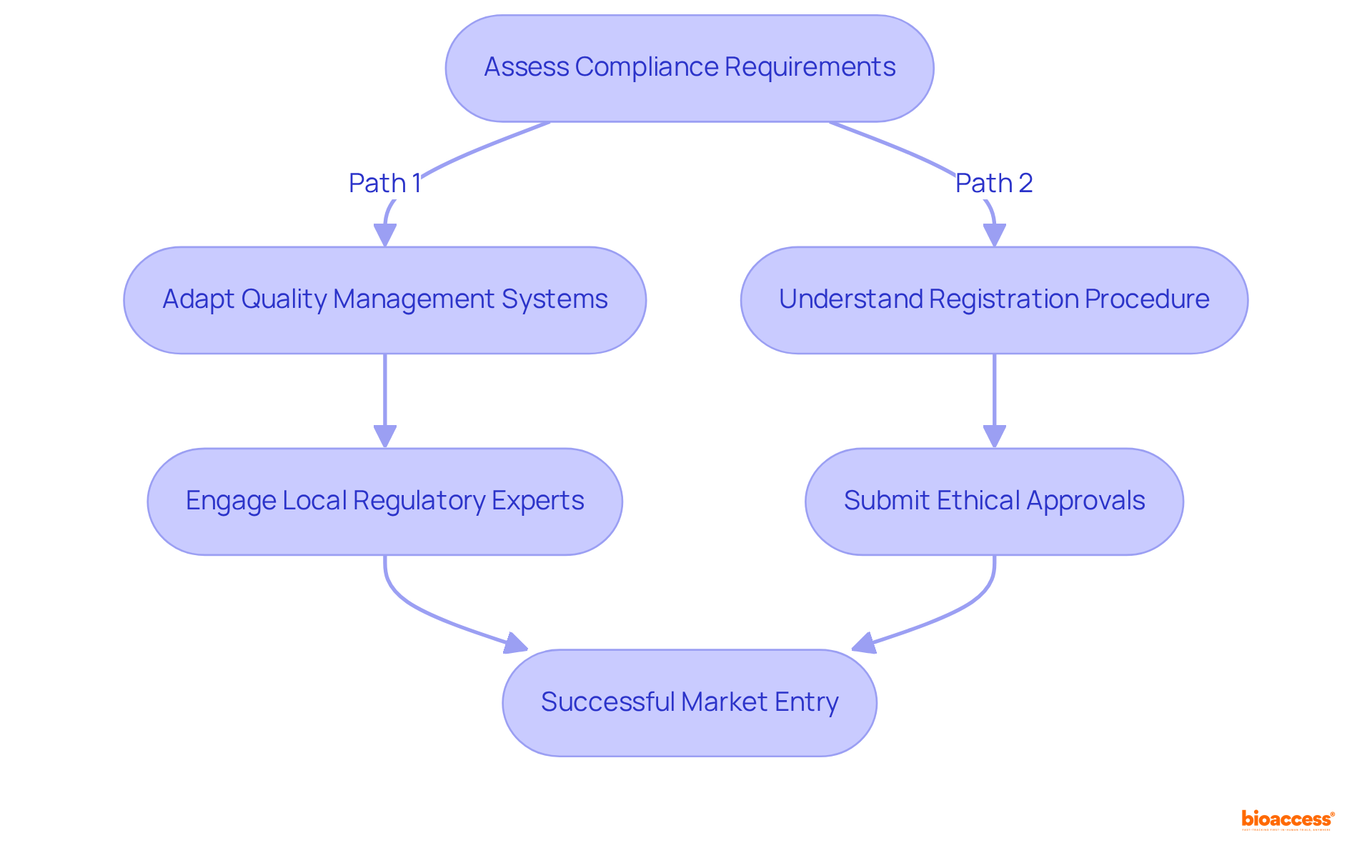
Navigating the compliance environment in Mexico is essential for Medtech companies seeking successful entry into the industry. Adhering to the medical device GMP Mexico overview established by the regulatory authority requires companies to adjust their existing quality management systems to meet local regulations. This adaptation is vital, as imports constitute nearly 90% of medical devices sold in Mexico, with US manufacturers dominating over 50% of the import market. Understanding the registration procedure and its associated timelines is critical; ethical approvals may take 4-6 weeks, and delays in submissions can lead to backlogs that hinder timely access.
Engaging local regulatory experts represents a strategic initiative that can enhance communication with COFEPRIS, effectively navigating the complexities of the approval process. These specialists provide insights into the nuances of local regulations, significantly increasing the likelihood of successful market entry. Statistics reveal that companies proactively addressing compliance challenges and utilizing local expertise enjoy higher success rates in penetrating the Mexican market, as highlighted in the medical device GMP Mexico overview, which is projected to grow at an annual rate of 5.93% from 2025 to 2030, ultimately reaching a market volume of US$11.79 billion by 2030. By prioritizing these strategies, Medtech companies can secure a robust foothold in Mexico's expanding healthcare sector.
The landscape of Good Manufacturing Practices (GMP) for medical devices in Mexico is evolving significantly, particularly with the recent updates by COFEPRIS through NOM-241-SSA1-2025. This comprehensive framework not only enhances quality and safety standards but also streamlines market access for manufacturers. By mandating GMP certification for all medical devices, including low-risk products, the new regulations ensure that manufacturers adhere to stringent quality management systems, ultimately benefiting patient safety and product efficacy.
Key insights from the article highlight the alignment of Mexican GMP with global standards, such as ISO 13485, promoting a culture of quality through rigorous documentation, audits, and continuous improvement. However, the article also emphasizes important distinctions, including specific sanitary registration requirements and the local manufacturing focus that foreign companies must navigate. Understanding these nuances is critical for Medtech firms aiming to penetrate the Mexican market successfully, particularly as the sector is projected to grow significantly in the coming years.
In conclusion, the implications of these regulatory changes are profound for Medtech companies. Engaging local regulatory experts and adapting quality management systems to meet Mexico's unique requirements will be essential for successful market entry. As the medical device sector continues to expand, proactive measures in compliance will not only enhance operational efficiency but also foster credibility and competitiveness in both local and international markets. Embracing these strategies will be vital for stakeholders aiming to thrive in Mexico's dynamic healthcare environment.
What organization regulates Good Manufacturing Practices (GMP) for medical devices in Mexico?
The Federal Commission for the Protection Against Sanitary Risks (COFEPRIS) regulates GMP for medical devices in Mexico.
What is the new standard that updates the GMP guidelines in Mexico?
The new standard is NOM-241-SSA1-2025, which provides an overview of medical device GMP in Mexico.
What are the key requirements outlined in the medical device GMP Mexico overview?
Key requirements include sanitary registration, adherence to quality management systems, and mandatory GMP certification for all medical devices, including low-risk products, effective from November 30, 2025.
Who must comply with the new GMP regulations in Mexico?
Only entities that manufacture, condition, store, or distribute medical devices for commercialization in Mexico must comply, excluding contract manufacturers whose products are sold abroad.
How do the new regulations enhance patient safety and market access?
The strategic approach of the regulations enhances patient safety and product efficacy while streamlining market access for manufacturers.
What internationally accepted documents does COFEPRIS now acknowledge as part of the GMP overview?
COFEPRIS acknowledges MDSAP Audit Certificates and CE Mark Certificates as part of the medical device GMP Mexico overview to expedite compliance and approval processes.
Has COFEPRIS provided additional resources for stakeholders regarding the new standard?
Yes, COFEPRIS has published an accompanying FAQ guideline to address common inquiries related to the new standard.
What is the purpose of the updates to the GMP regulations?
The updates aim to reduce complexity and improve transparency for stakeholders in the medical device sector.