


The article delineates the two primary registration types for medical items in Mexico:
Ordinary Registration is essential for new products that necessitate thorough evaluation, while Similar Registration facilitates expedited approval for items that are comparable to existing products. This differentiation is crucial, as it significantly influences the time, cost, and regulatory strategies for companies. Specifically, Ordinary Registration is characterized by longer timelines and higher costs, whereas Similar Registration provides quicker market access with reduced documentation requirements.
In the intricate landscape of medical device approvals in Mexico, grasping the nuances between Ordinary and Equivalence registration types is essential for industry stakeholders. These two distinct pathways not only dictate the timeline and cost of bringing products to market but also shape the overall strategy for regulatory compliance.
As firms navigate these options, a pressing question arises: how can companies balance the necessity for thorough evaluation with the urgency for swift market entry?
This article explores the fundamental differences between these registration types, providing insights that can optimize the enrollment process and enhance competitive positioning in a rapidly evolving market.
In Mexico, the registration of medical items is overseen by the Federal Commission for the Protection against Sanitary Risk (COFEPRIS), which outlines the Mexico registration types ordinary vs equivalence as two primary registration pathways: Ordinary and Similar.
Ordinary Registration is essential for new medical devices or pharmaceuticals that have not previously received approval in Mexico. This process necessitates a thorough evaluation of the item's safety, efficacy, and quality, typically extending over several months. The standard review period for Ordinary Registration can span from 3 to 8 months, with a validity of 5 years, which is renewable if no significant changes occur.
Conversely, Similarity Registration is designed for items that are akin to those already authorized in Mexico. This route enables a more expedited review process, utilizing existing data from comparable products. The Comparable pathway is particularly advantageous for firms aiming for swift entry into the industry with established technologies, as it can reduce approval time to as short as 1 to 3 months when leveraging an Authorized Third Party.
Looking ahead to 2025, the landscape of medical device approvals in Mexico is expected to reflect a growing trend towards Equivalence approvals, driven by the demand for quicker access to the market. Regulatory experts underscore the significance of adeptly navigating these pathways, as they profoundly affect regulatory strategies and market entry timelines for stakeholders in the Medtech and Biopharma sectors. Understanding the distinctions related to Mexico registration types ordinary vs equivalence is crucial for optimizing the enrollment process and ensuring compliance with COFEPRIS regulations.
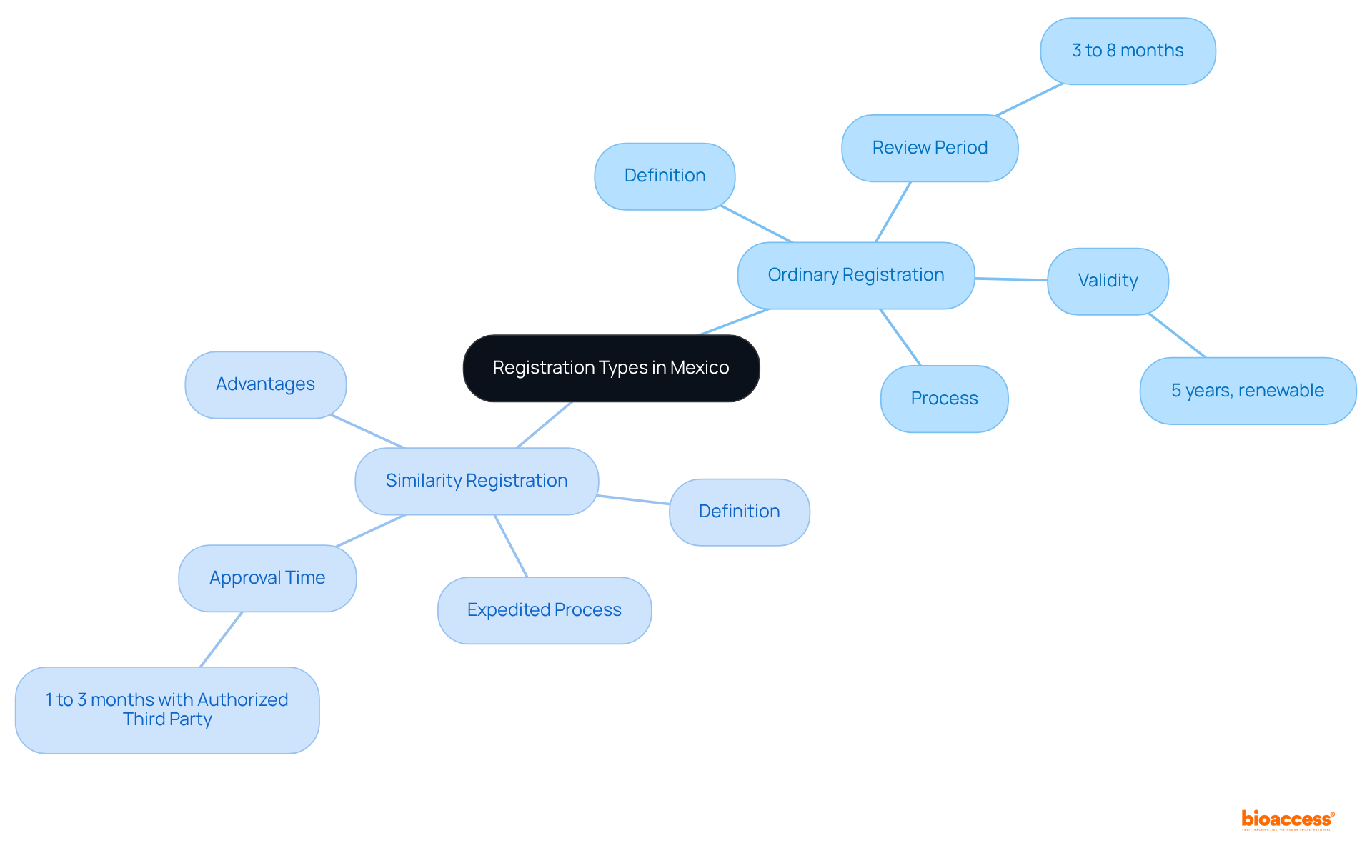
When evaluating the different Mexico registration types ordinary vs equivalence, several essential factors come into play.
Timeframe: Standard sign-ups frequently take longer than six months due to a comprehensive assessment procedure. In contrast, the Mexico registration types ordinary vs equivalence show that equivalency sign-ups can be finalized in just two to three months, rendering them a more appealing choice for businesses pursuing swift market access.
Cost: The financial implications of standard enrollments are typically higher, driven by extensive documentation and testing requirements. Conversely, when considering Mexico registration types ordinary vs equivalence, equivalency certifications are generally more economical, as they demand less stringent documentation.
Data Requirements: Standard submissions necessitate thorough clinical information to validate safety and effectiveness. In contrast, submissions under Mexico registration types ordinary vs equivalence utilize available data from comparable items, significantly reducing the applicant's load.
Market Impact: The decision regarding enrollment categories can profoundly affect an item's positioning and competitive advantage, influencing pricing tactics and promotional methods.
These standards serve as a valuable framework for identifying the most appropriate enrollment type, specifically the Mexico registration types ordinary vs equivalence, based on the specific characteristics of the item and the company's goals.
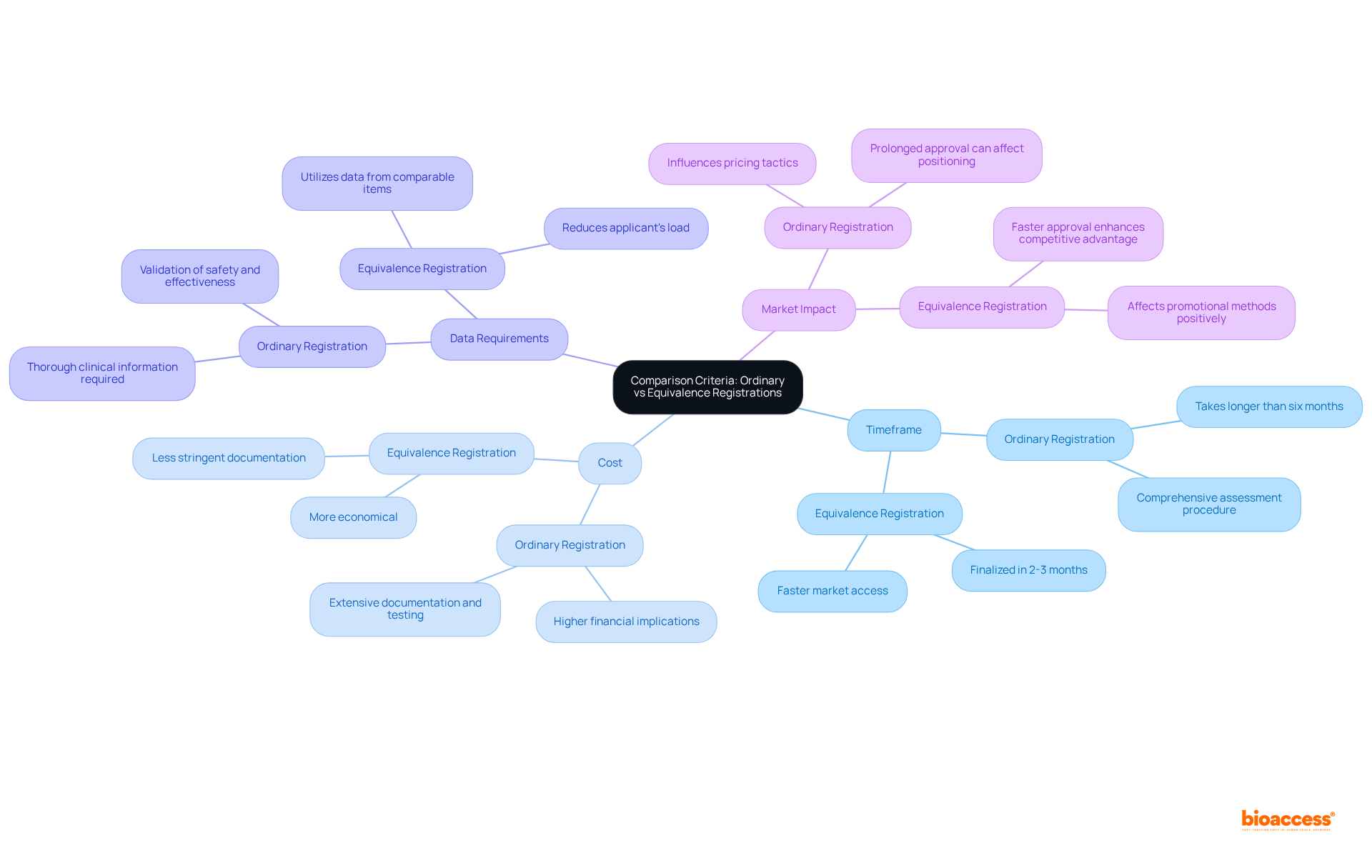
Companies must consider the distinct advantages and disadvantages presented by the Mexico registration types ordinary vs equivalence.
Ordinary Registration offers significant benefits. A comprehensive evaluation enhances product credibility and can significantly improve market acceptance rates, as supported by bioaccess's feasibility studies and compliance reviews. This pathway is ideal for innovative products that necessitate extensive validation, ensuring thorough scrutiny of safety and efficacy through meticulous trial setup and project management. However, companies must also be aware of the drawbacks. The lengthy approval process can delay market entry, potentially allowing competitors to gain an advantage. Additionally, higher costs associated with extensive data requirements can strain budgets, especially for startups.
On the other hand, Equivalence Registration provides a different set of pros and cons. Faster approval times facilitate quicker market access, enabling companies to capitalize on emerging opportunities more swiftly, aided by streamlined processes in clinical trial management. Furthermore, lower costs due to reduced data requirements make this pathway attractive for many Medtech firms, particularly those with limited resources. Conversely, this pathway is limited to products that can demonstrate equivalence to existing products, which may stifle innovation and the introduction of novel solutions. Moreover, a potentially less rigorous assessment process may raise concerns regarding item safety and efficacy, impacting long-term consumer trust and underscoring the importance of thorough project management and monitoring.
Grasping these advantages and disadvantages is essential for companies to align their enrollment strategy with development objectives and consumer needs, particularly regarding Mexico registration types ordinary vs equivalence. As the medical devices market in Mexico is expected to attain USD 7.6 billion by 2025, making informed choices regarding types of approval can greatly impact a product's success.
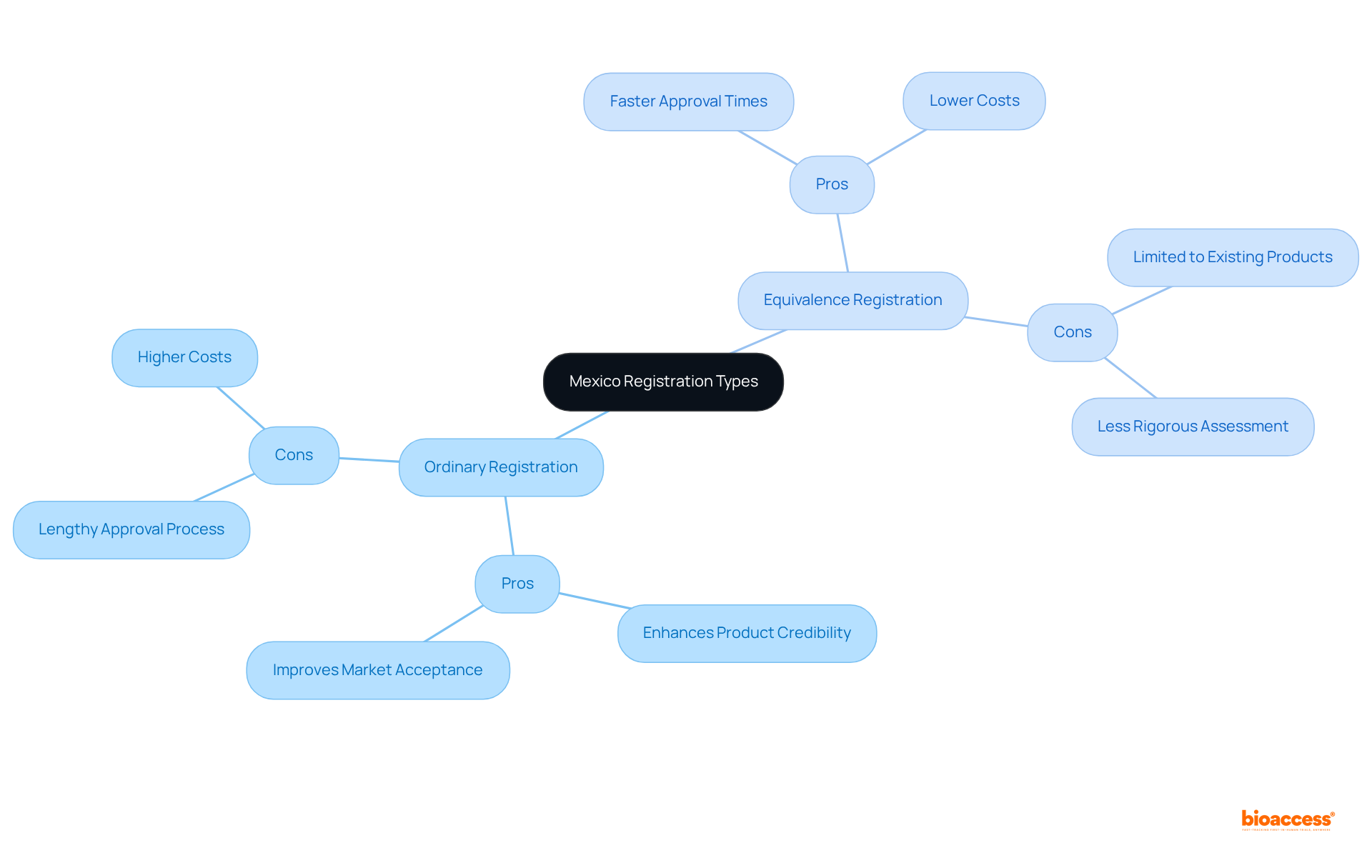
The decision regarding Mexico registration types ordinary vs equivalence carries substantial implications for clinical research.
Study Design: Standard enrollments typically necessitate more comprehensive clinical trials to produce the requisite data, significantly influencing the design and scope of studies. In contrast, Mexico registration types ordinary vs equivalence may facilitate streamlined studies that leverage existing data, thereby reducing the time and resources required for completion.
Regulatory Strategy: It is imperative for companies to align their clinical research strategies with their selected approval type. For example, those pursuing Mexico registration types ordinary vs equivalence enrollment may need to allocate resources toward more extensive preclinical studies to strengthen their applications, ensuring compliance with regulatory expectations.
Product Preparedness: The speed of sign-up directly impacts a product's readiness for launch. Businesses opting for Mexico registration types ordinary vs equivalence certification can benefit from expedited timelines, allowing them to respond more swiftly to industry demands and seize new opportunities.
Risk Management: A thorough understanding of the regulatory landscape and the implications associated with each approval type is vital for mitigating risks linked to delays or unforeseen regulatory challenges. This proactive strategy can protect against potential setbacks that may impede product development and market entry.
In conclusion, the selection of registration type should be guided by a comprehensive understanding of these factors, ensuring that clinical research initiatives are strategically aligned with overarching business objectives.
Navigating the registration landscape for medical devices and pharmaceuticals in Mexico is essential for companies looking to penetrate this dynamic market. Understanding the distinctions between Ordinary and Equivalence registrations can significantly influence strategic decisions and market readiness. While Ordinary Registration provides a comprehensive assessment of new products, ensuring safety and efficacy, Equivalence Registration offers a faster, more cost-effective route for items similar to those already approved.
Key insights from the article emphasize the varying timeframes, costs, and data requirements associated with each registration type:
These factors collectively underscore the importance of aligning registration strategies with product development goals and market demands.
As the Mexican medical device market is projected to reach USD 7.6 billion by 2025, the implications of selecting the appropriate registration type extend beyond mere compliance. Companies are encouraged to carefully evaluate their options, weighing the advantages and disadvantages of each pathway. By doing so, they can optimize their clinical research initiatives, manage risks effectively, and ultimately enhance their competitive edge in a rapidly evolving industry.
What are the two primary registration pathways for medical items in Mexico?
The two primary registration pathways for medical items in Mexico are Ordinary Registration and Similarity Registration.
What is Ordinary Registration in Mexico?
Ordinary Registration is required for new medical devices or pharmaceuticals that have not previously received approval in Mexico. It involves a thorough evaluation of the item's safety, efficacy, and quality, typically taking between 3 to 8 months to complete.
How long is the validity of Ordinary Registration, and can it be renewed?
The validity of Ordinary Registration is 5 years, and it can be renewed if no significant changes occur.
What is Similarity Registration, and who is it designed for?
Similarity Registration is designed for items that are similar to those already authorized in Mexico. It allows for a more expedited review process by utilizing existing data from comparable products.
How does the approval time differ between Ordinary and Similarity Registration?
The approval time for Ordinary Registration can take 3 to 8 months, while Similarity Registration can reduce approval time to as short as 1 to 3 months, especially when leveraging an Authorized Third Party.
What trend is expected in medical device approvals in Mexico by 2025?
By 2025, there is an expected trend towards increased Equivalence approvals, driven by the demand for quicker access to the market.
Why is it important to understand the distinctions between Ordinary and Similarity Registration?
Understanding the distinctions between Ordinary and Similarity Registration is crucial for optimizing the enrollment process and ensuring compliance with COFEPRIS regulations, which can significantly affect regulatory strategies and market entry timelines for stakeholders in the Medtech and Biopharma sectors.