


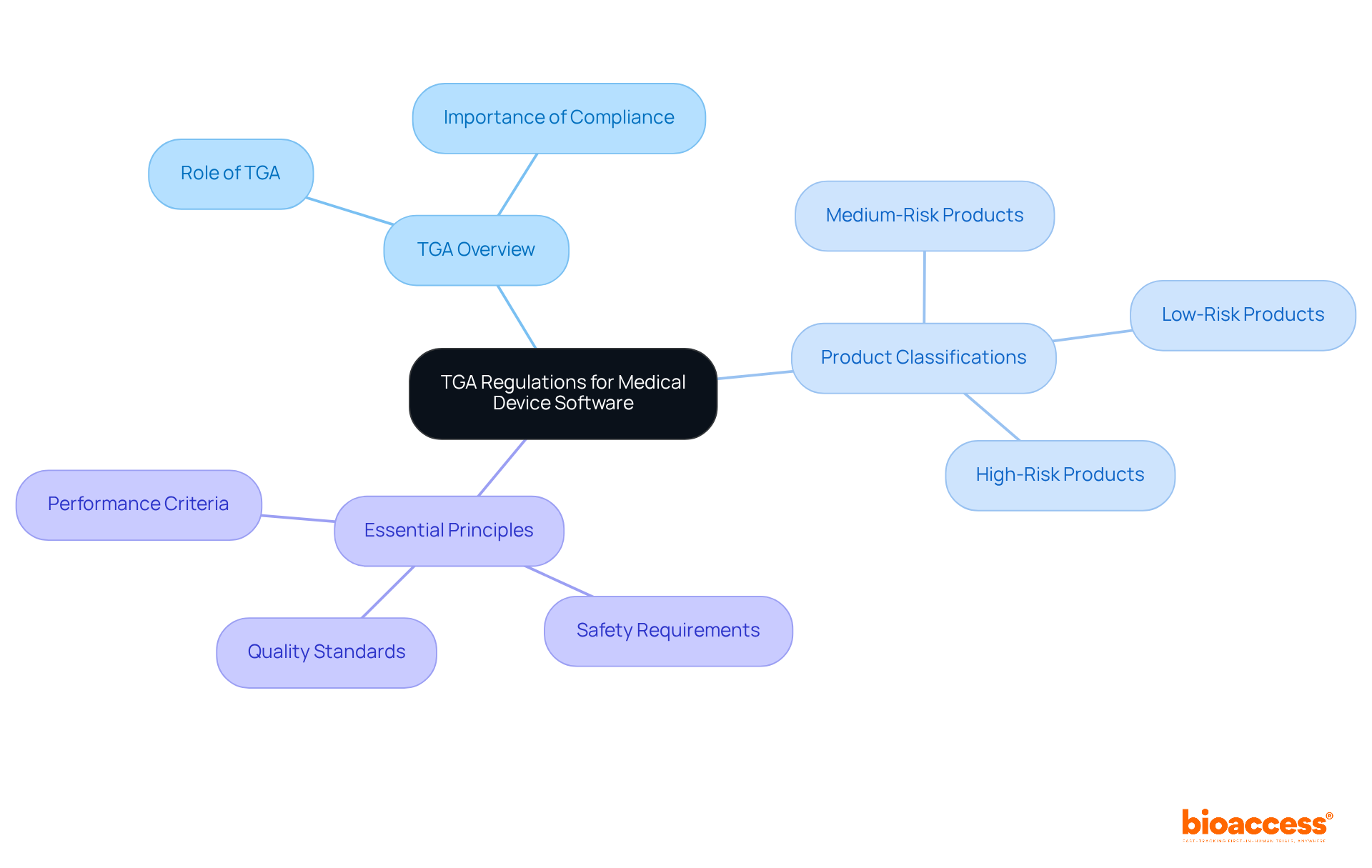
Navigating Australia TGA regulations is essential for the success of medical devices. A thorough understanding of these regulations not only ensures compliance with safety and performance standards but also enhances the likelihood of successful market entry. Familiarizing oneself with the TGA's classification system and Essential Principles is paramount. Moreover, adhering to best practices for documentation and communication plays a critical role in mitigating common pitfalls. Collectively, these strategies streamline the approval process and position stakeholders for success in the Medtech landscape.
Navigating the intricate landscape of medical device regulations is no small feat, especially in Australia, where the Therapeutic Goods Administration (TGA) plays a pivotal role in ensuring product safety and efficacy. Understanding TGA regulations is not merely a formality; it is a critical step that can ultimately dictate the success or failure of medical device software in a competitive market. Given the complexities of compliance requirements and the potential for costly missteps, manufacturers must ask: how can they streamline their processes to achieve timely approvals? This guide explores essential strategies for mastering TGA regulations, equipping readers with the knowledge necessary to avoid common pitfalls and enhance their chances of success.
The Therapeutic Goods Administration (TGA), also referred to as Australia TGA, serves as the authoritative body overseeing medical equipment in Australia. Understanding the Australia TGA's regulations is essential for ensuring your medical product adheres to the necessary safety and performance standards.
The TGA classifies medical products into different categories based on their risk levels, which directly influences the compliance pathway you must navigate. It is imperative to familiarize yourself with the Essential Principles set forth by Australia TGA, which outline requirements for safety, quality, and performance. This foundational knowledge will guide your subsequent actions in the compliance process and help you avoid common pitfalls that may delay your product's approval.
Experts like Ana Criado, with her extensive experience in compliance and biomedical engineering, can provide invaluable insights into effectively navigating these regulations. Additionally, Katherine Ruiz's expertise in regulatory matters for medical equipment and in vitro diagnostics underscores the importance of grasping the Australia TGA's framework.
For further detailed information, refer to the TGA's official guidelines on medical equipment here.
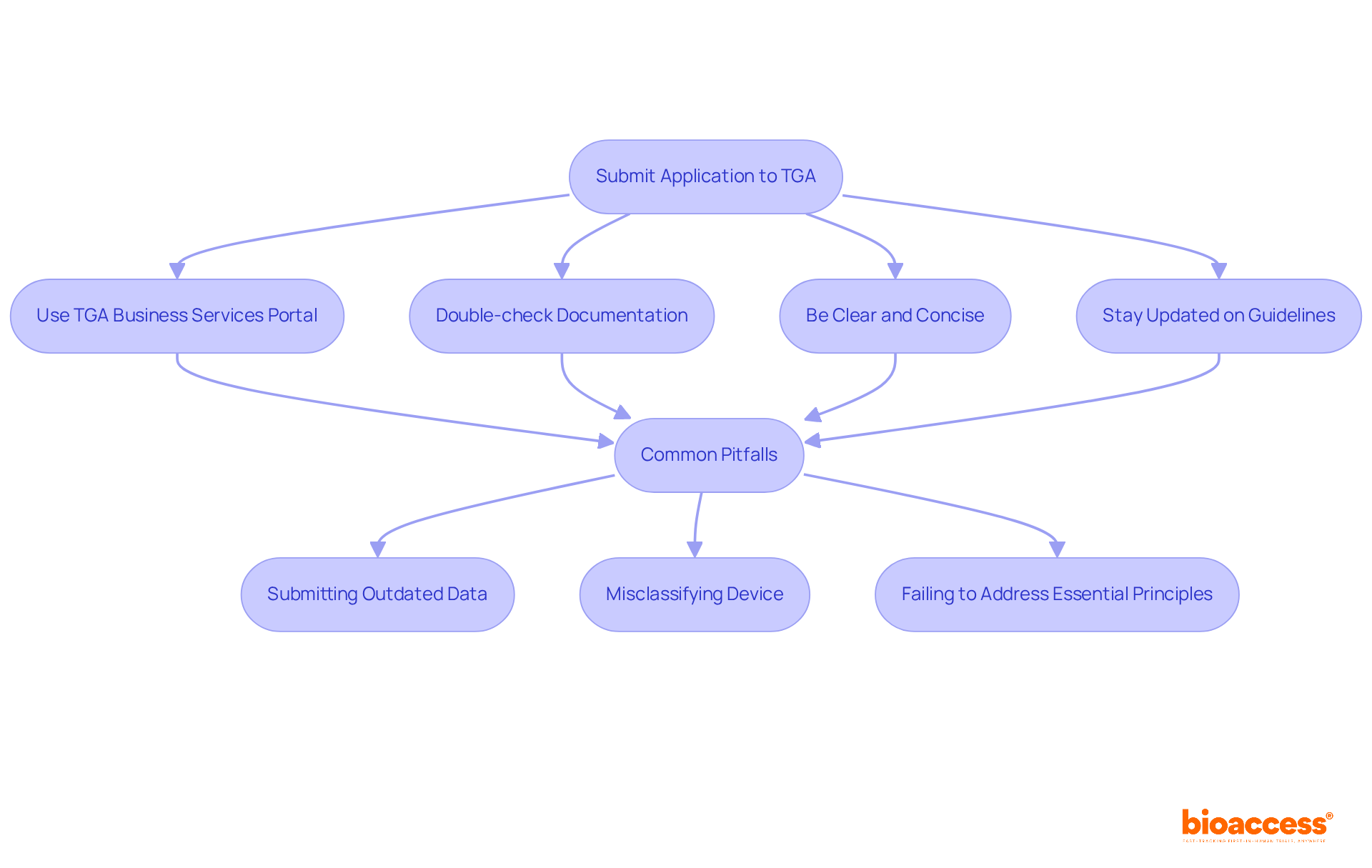
Before submitting your application to the Australia TGA, it is imperative to gather all required documentation. This typically includes:
Conducting an initial assessment of your documentation is crucial to ensure completeness and compliance with Australia TGA requirements. This proactive strategy assists in recognizing any gaps or issues prior to submission, thereby minimizing the chances of delays in the approval phase. With bioaccess®'s comprehensive clinical trial management services, including site selection and compliance reviews, you can effectively navigate the complexities of the regulatory landscape and achieve successful outcomes.

When submitting your application to the Australia TGA, it is essential to adhere to best practices that can streamline the process and enhance your chances of success.
Leveraging comprehensive clinical trial management services can significantly enhance your submission process. Services such as feasibility studies, site selection, compliance assessments, trial setup, import permits, project management, and reporting directly support these best practices by ensuring thorough preparation and adherence to legal standards.
Common pitfalls to avoid include:
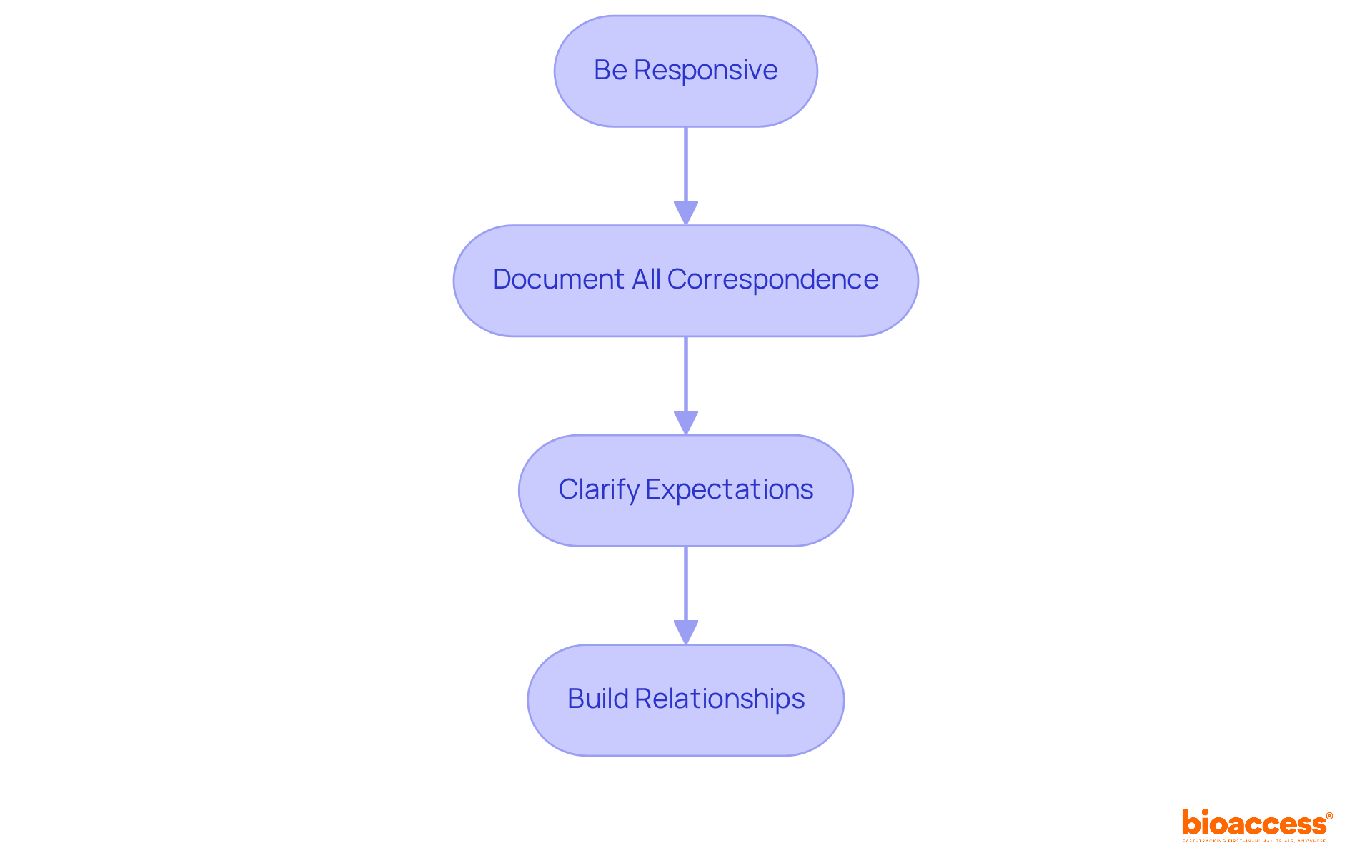
Once your application is submitted, it is crucial to maintain open lines of communication with the TGA. Effective management of these communications can significantly influence the approval process.
Be Responsive: When the TGA requests additional information or clarification, respond promptly and thoroughly. Delays in communication can prolong the approval process, which can be detrimental to your objectives.
Document All Correspondence: Keep a meticulous record of all communications with the TGA, including inquiries and responses. This documentation serves as a valuable resource for future reference and can aid in tracking the progress of your application.
Clarify Expectations: Upon receiving feedback or requests from the TGA, ensure you fully understand their expectations. Do not hesitate to seek clarification if needed; this will help you align your responses with their requirements.
Build Relationships: Establishing a positive rapport with TGA representatives can facilitate smoother interactions. Approach all communications with professionalism and respect, as these relationships can prove beneficial in navigating regulatory challenges.
By managing communications effectively, you can navigate the regulatory landscape more efficiently and enhance the likelihood of a successful outcome.
Navigating the Australia TGA regulations is essential for the successful approval of medical devices. Understanding the TGA's framework not only ensures compliance with safety and performance standards but also significantly enhances the likelihood of a smooth application process. By familiarizing themselves with the classification of medical products and the Essential Principles, manufacturers can strategically position their products for success in the competitive medical device market.
Key points include:
Engaging experts and utilizing specialized services can further streamline the process, helping to avoid common pitfalls that could delay approval. Effective communication with the TGA is also pivotal; maintaining responsiveness and clarity can greatly influence the outcome of the application.
Ultimately, understanding and adhering to TGA regulations transcends mere compliance; it fosters innovation and ensures that safe, effective medical devices reach those in need. Manufacturers are encouraged to take proactive steps in their regulatory journey, leveraging available resources and expertise to navigate the complexities of TGA compliance successfully. Embracing these practices not only enhances individual product success but also contributes to the overall advancement of healthcare in Australia.
What is the role of the Therapeutic Goods Administration (TGA) in Australia?
The TGA is the authoritative body overseeing medical equipment in Australia, ensuring that medical products adhere to necessary safety and performance standards.
Why is it important to understand TGA regulations for medical device software?
Understanding TGA regulations is essential to ensure that your medical product complies with the required safety and performance standards, facilitating a smoother approval process.
How does the TGA classify medical products?
The TGA classifies medical products into different categories based on their risk levels, which directly influences the compliance pathway that must be navigated.
What are the Essential Principles set forth by the TGA?
The Essential Principles are requirements outlined by the TGA that address safety, quality, and performance standards for medical products.
Who can provide insights into navigating TGA regulations?
Experts like Ana Criado, who has extensive experience in compliance and biomedical engineering, and Katherine Ruiz, who specializes in regulatory matters for medical equipment and in vitro diagnostics, can provide valuable insights.
Where can I find more detailed information about TGA regulations?
For further detailed information, you can refer to the TGA's official guidelines on medical equipment.