


Navigating the complex landscape of clinical research demands a sharp understanding of the evolving guidelines set forth by the US FDA. These recent updates not only underscore the critical importance of participant safety and ethical conduct but also present a strategic opportunity for researchers to bolster their operational efficiency and enhance market credibility.
However, with the introduction of stricter compliance measures and potential penalties for non-adherence, researchers face a pressing question: how can they effectively align their studies with these new regulations while ensuring the integrity of their work?
This challenge is not just a regulatory hurdle; it’s a pivotal moment for researchers to rethink their strategies and embrace the changes that lie ahead.
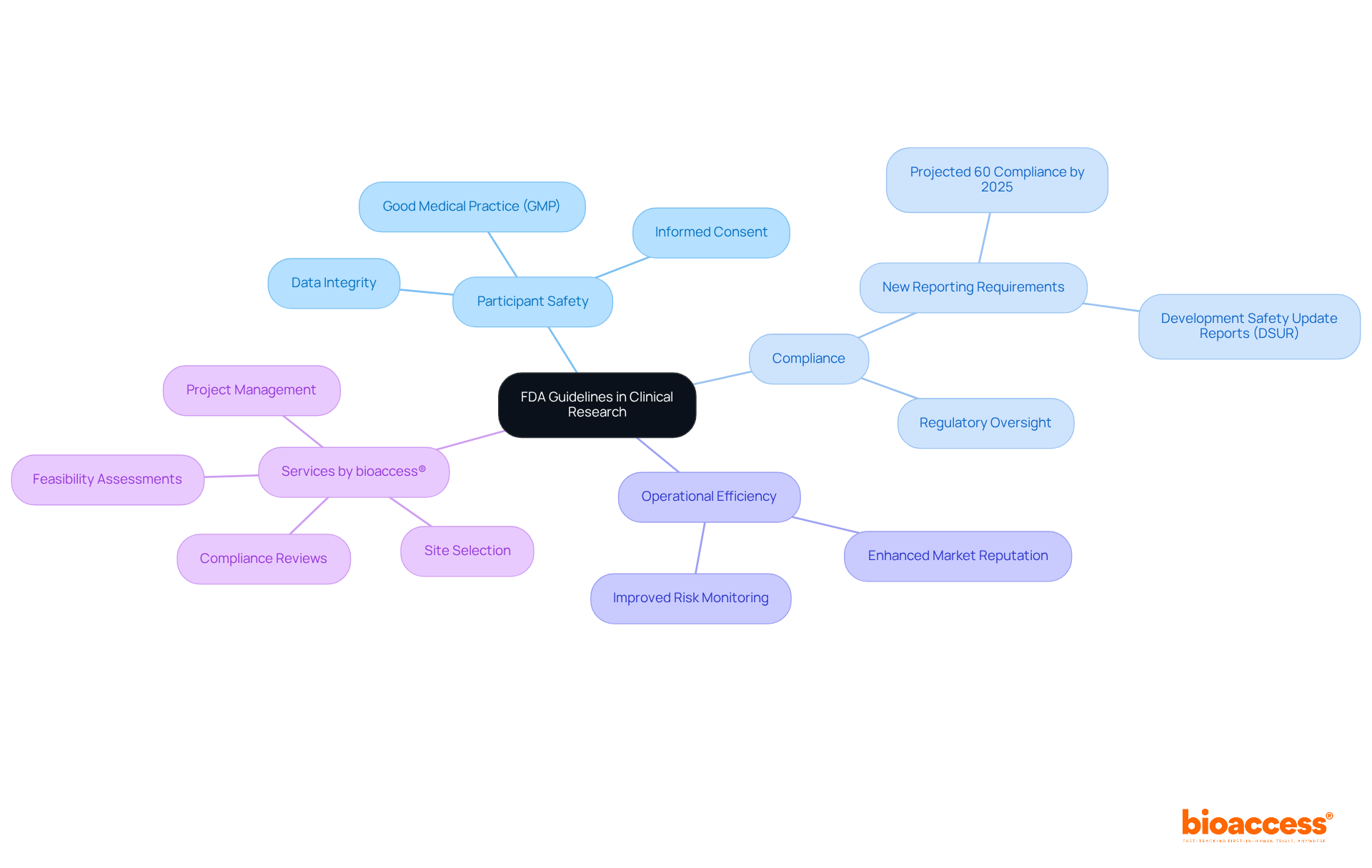
The recent US FDA guidelines establish a comprehensive framework for conducting research on humans in the United States, setting essential benchmarks for study design, execution, and documentation. These guidelines prioritize participant safety and ethical conduct, focusing on critical areas such as good medical practice (GMP), informed consent, and data integrity. For example, the shift to Development Safety Update Reports (DSURs) has notably enhanced the identification of serious adverse events, thereby improving participant safety and regulatory oversight.
Compliance with the recent US FDA guidelines is not just a regulatory obligation; it is a strategic necessity that can enhance operational efficiency and bolster market reputation. Statistics reveal that around 60% of sponsors are projected to meet the new reporting requirements by 2025, highlighting a growing acknowledgment of the importance of adhering to FDA standards. Furthermore, expert opinions stress that maintaining rigorous ethical standards in research fosters public trust and confidence in medical investigations.
By ensuring that studies are both scientifically valid and ethically conducted, FDA oversight plays a pivotal role in streamlining the approval process for new medical products, ultimately benefiting patient care and driving healthcare innovation. In this landscape, bioaccess® offers a range of comprehensive study management services. These include:
By leveraging these tailored services, Medtech, Biopharma, and Radiopharma startups can accelerate their research efforts, effectively navigate regulatory challenges, and enhance their chances of success in a competitive market.
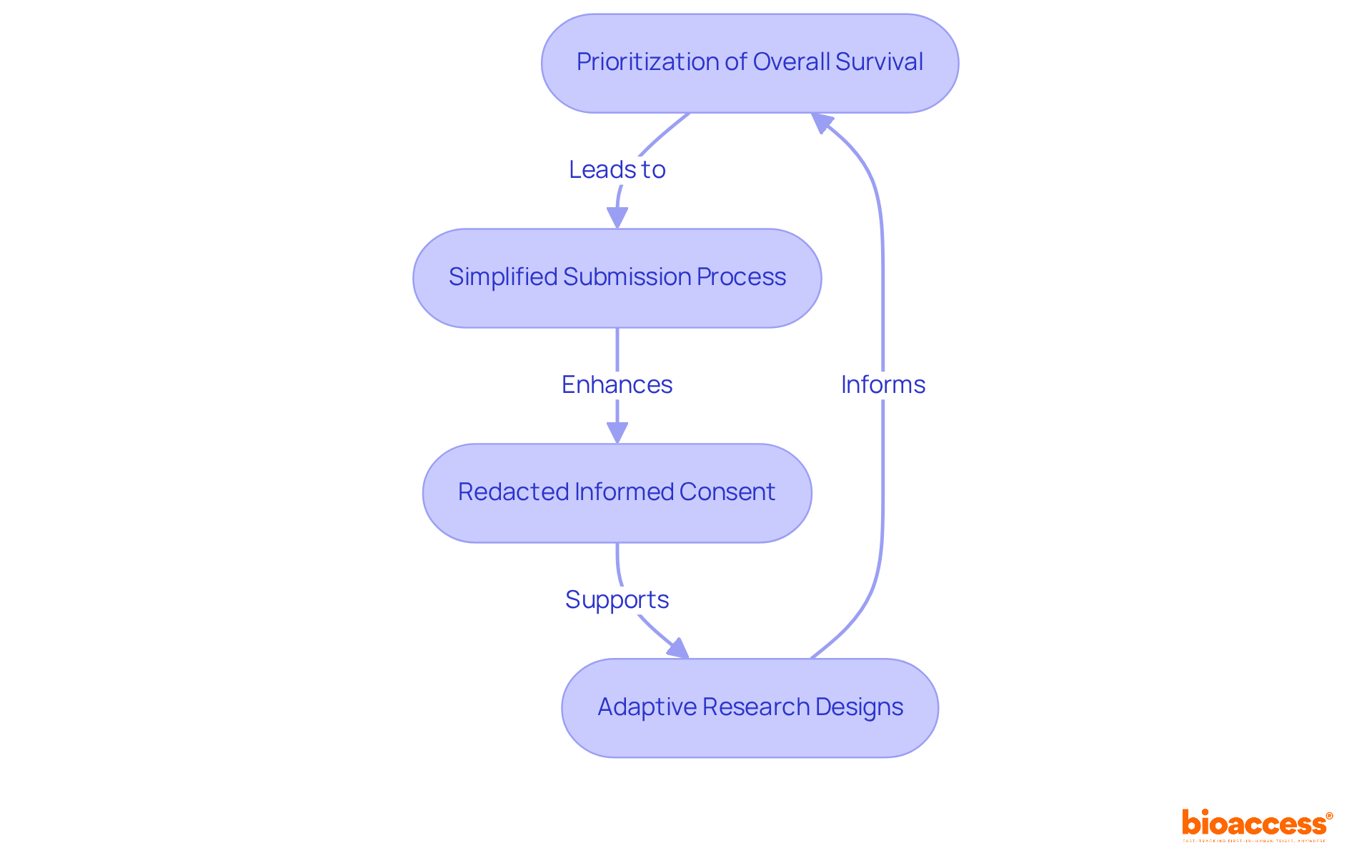
In 2025, the FDA made significant updates to its clinical study guidelines, reflecting the recent US FDA guidelines and marking a pivotal shift towards adaptive and patient-centered methodologies. A key focus is the prioritization of overall survival as a primary endpoint in oncology studies, aligning with the agency's commitment to enhancing treatment efficacy evaluation. The introduction of new guidance on regenerative medicine therapies further underscores the FDA's dedication to fostering innovation in this rapidly evolving field.
Moreover, the FDA has simplified the submission process for study data and results, shortening the timeline for sponsors to report findings from 12 months to just 9 months after the primary completion date. This change aims to enhance transparency and accountability, ensuring that critical health data is accessible to the scientific community and the public in a timely manner.
Furthermore, all relevant research studies (ACTs) are now required to submit redacted versions of their informed consent documents, reflecting a significant update aimed at improving transparency and patient focus in research communications.
To navigate these changes effectively, researchers can leverage bioaccess®'s comprehensive service capabilities, which include:
These services enable treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than traditional Western sites. This acceleration not only addresses patient recruitment challenges but also results in significant cost savings of $25K per patient, thanks to bioaccess's FDA-ready data that eliminates rework and delays.
Researchers must adjust their study designs to align with these updates, particularly in the context of adaptive research designs that permit modifications based on interim results. For instance, concurrently managed superiority studies are now acknowledged as the most stringent design for assessing both human and animal medications, highlighting the necessity for robust methodologies in research involving patients.
As the regulatory landscape evolves, staying informed about these changes is essential for maintaining the integrity of medical research and ensuring compliance. The recent US FDA guidelines emphasize the increased scrutiny on data integrity and the introduction of stricter penalties for noncompliance, which can reach $15,000 per day, underscoring the importance of adherence. By adopting these updates and utilizing extensive study management services from bioaccess®, researchers can enhance their study designs, ultimately leading to better patient outcomes and increased trust in the research process.
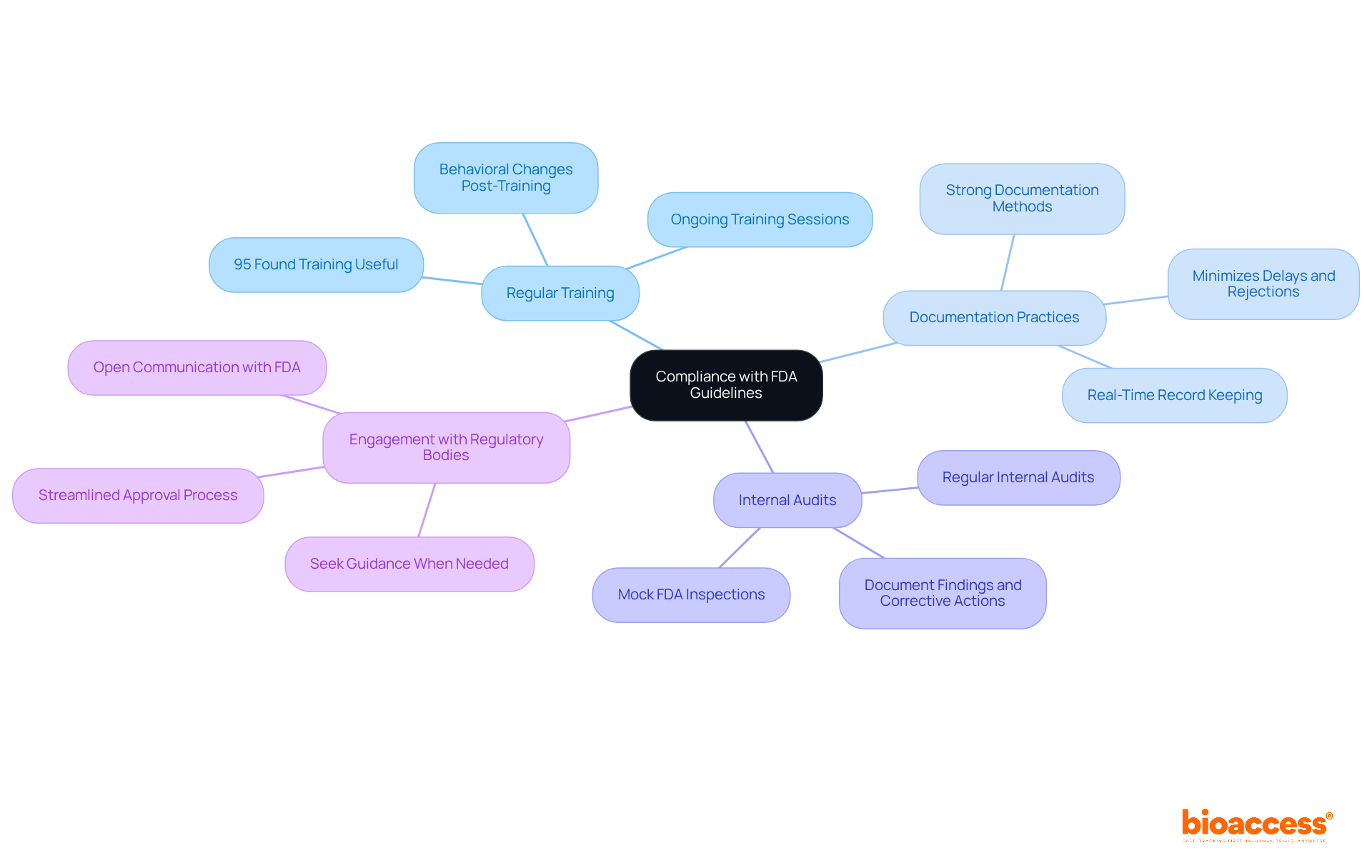
To ensure compliance with updated FDA guidelines, researchers must adopt several key strategies:
Regular Training: Conduct ongoing training sessions for all team members on the latest FDA regulations and guidelines. This guarantees that all participants in the examination comprehend their duties and the essential nature of adherence. Studies show that 95% of participants found training useful for their study responsibilities, reinforcing its value in clinical settings.
Documentation Practices: Establish strong documentation methods to keep precise records of all study activities, including informed consent procedures, data gathering, and adverse event reporting. Efficient documentation is essential; organizations that emphasize it can greatly minimize the chance of delays or rejections in submissions due to inadequate traceability.
Internal Audits: Schedule regular internal audits to assess compliance with FDA guidelines and identify areas for improvement. Conducting mock FDA inspections at least twice a year can enhance preparedness and ensure that all documentation is bulletproof, making it easily retrievable during actual inspections, as recommended by recent US FDA guidelines.
Engagement with Regulatory Bodies: Foster open communication with FDA representatives and seek guidance when needed. Engaging with regulatory bodies can provide valuable insights and clarify uncertainties regarding compliance, ultimately leading to a more streamlined approval process.
By incorporating these strategies, researchers can improve their adherence efforts, reduce hazards, and contribute to the overall success of their trials.
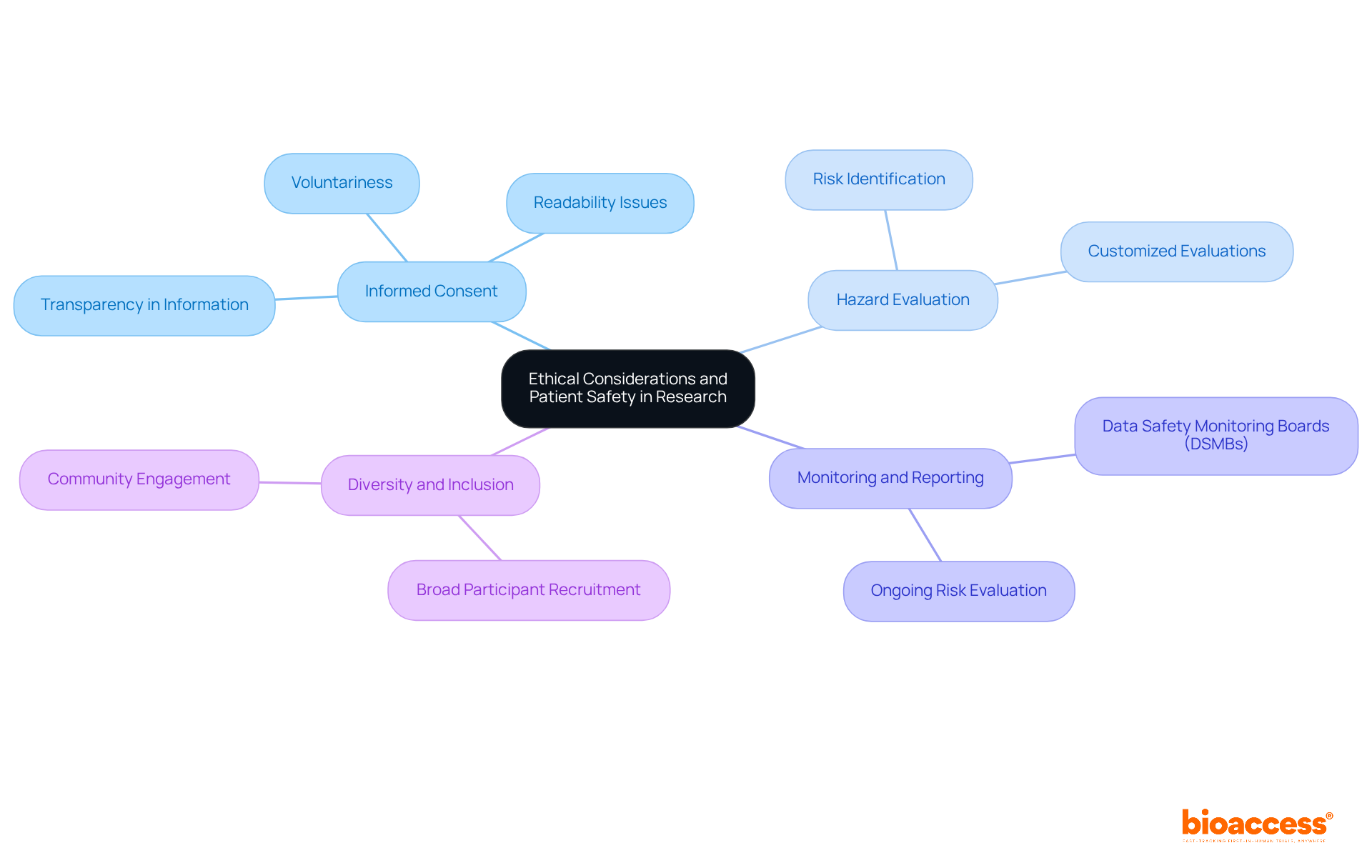
Prioritizing ethical considerations and patient safety is fundamental in clinical research. Researchers must adhere to the principles outlined in the Belmont Report, which emphasizes respect for persons, beneficence, and justice.
Key practices include:
By embedding these ethical considerations into the research process, investigators can enhance participant safety and uphold the integrity of clinical trials.
Navigating the recent US FDA guidelines is essential for the success of clinical research endeavors. These updated regulations establish crucial standards for study design and ethical conduct, while also emphasizing participant safety and data integrity. By adhering to these guidelines, researchers can enhance operational efficiency and build a reputable standing in the medical community, ultimately leading to improved patient care and accelerated healthcare innovations.
Key insights discussed throughout the article underscore the significance of compliance with the latest FDA requirements. The implementation of Development Safety Update Reports (DSURs) and the simplification of study data submissions are designed to foster transparency and accountability in clinical trials. Moreover, adopting strategic practices such as regular training, robust documentation, and engaging with regulatory bodies can significantly bolster adherence efforts, ensuring that research is conducted ethically and effectively.
In light of these developments, it is imperative for researchers to remain vigilant and proactive in understanding and integrating the FDA guidelines into their study protocols. By prioritizing ethical considerations and patient safety, researchers not only uphold the integrity of their work but also contribute to a more trustworthy and efficient clinical research landscape. Embracing these guidelines and leveraging comprehensive study management services can empower researchers to navigate regulatory challenges and ultimately enhance the success of their clinical trials.
What is the purpose of the recent US FDA guidelines in clinical research?
The recent US FDA guidelines establish a framework for conducting research on humans in the United States, focusing on participant safety, ethical conduct, study design, execution, and documentation.
What are some critical areas emphasized by the FDA guidelines?
The FDA guidelines prioritize areas such as good medical practice (GMP), informed consent, and data integrity, with an emphasis on enhancing participant safety and regulatory oversight.
How have Development Safety Update Reports (DSURs) impacted clinical research?
The shift to Development Safety Update Reports (DSURs) has improved the identification of serious adverse events, thereby enhancing participant safety and regulatory oversight.
Why is compliance with FDA guidelines considered a strategic necessity?
Compliance with FDA guidelines is seen as a strategic necessity because it enhances operational efficiency, bolsters market reputation, and fosters public trust in medical investigations.
What percentage of sponsors are expected to meet the new reporting requirements by 2025?
Approximately 60% of sponsors are projected to meet the new reporting requirements by 2025, indicating a growing recognition of the importance of adhering to FDA standards.
How does FDA oversight benefit patient care and healthcare innovation?
By ensuring studies are scientifically valid and ethically conducted, FDA oversight streamlines the approval process for new medical products, ultimately benefiting patient care and driving healthcare innovation.
What services does bioaccess® offer to support clinical research?
Bioaccess® offers a range of study management services including feasibility assessments, site selection, compliance reviews, efficient setup, import permits for investigational devices, project management, and thorough reporting on study progress and adverse events.
Who can benefit from the services provided by bioaccess®?
Medtech, Biopharma, and Radiopharma startups can benefit from bioaccess®'s services to accelerate their research efforts, navigate regulatory challenges, and enhance their chances of success in a competitive market.