


The article presents a comprehensive, step-by-step guide for navigating the Chinese NMPA approval process for medical devices. It clearly outlines essential steps, including:
Understanding regulatory requirements is crucial, as is collaborating with experts to effectively address challenges such as:
This guide serves as an invaluable resource for those seeking to navigate the intricacies of the approval process.
Navigating the labyrinthine landscape of medical device regulations in China presents a formidable challenge for any Medtech startup. The rapid expansion of the online medical equipment market, coupled with the increasing complexity of the NMPA approval process, underscores the critical importance of understanding the intricacies of this regulatory authority.
What steps must companies undertake to ensure compliance and secure approval? How can they effectively navigate the myriad challenges that arise along the way?
This guide offers a comprehensive roadmap for successfully maneuvering through the NMPA approval process, equipping businesses with the essential knowledge and tools required to thrive in this burgeoning market.
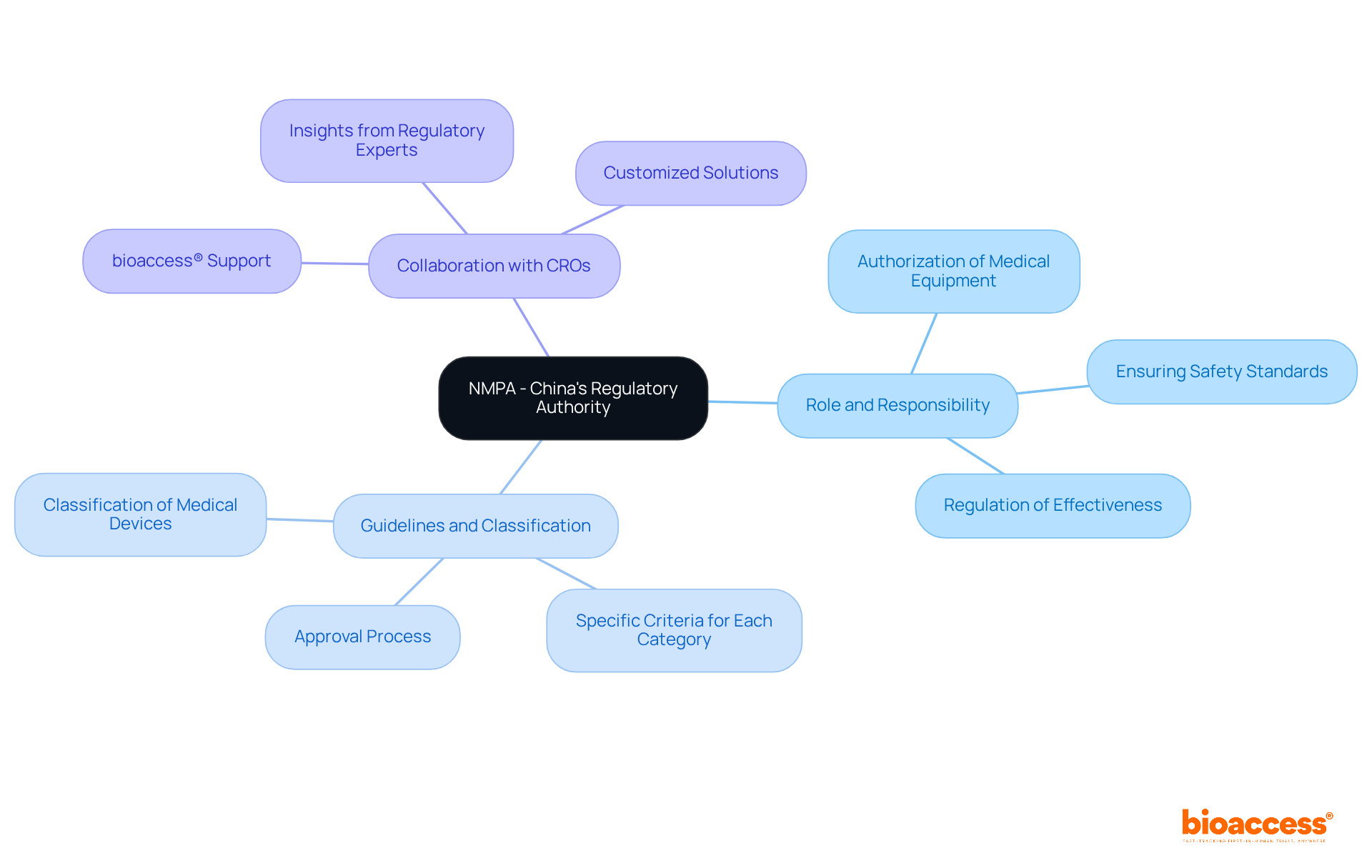
The Chinese NMPA serves as the governing authority in China, responsible for overseeing the authorization and regulation of medical equipment. Understanding the role of the Chinese NMPA is essential for any company seeking to enter the Chinese market, especially Medtech startups in pursuit of tailored solutions. This authority ensures that medical instruments meet the stringent safety and effectiveness standards established by the Chinese NMPA before they can be sold.
Familiarizing yourself with the Chinese NMPA's guidelines, including the classification of medical devices and the specific criteria for each category, is the crucial first step in the approval process. This foundational knowledge empowers you to navigate the complexities of the regulatory landscape, particularly the Chinese NMPA, and prepares you for the subsequent stages in obtaining permission.
Collaborating with a leading contract research organization (CRO) like bioaccess®, alongside insights from regulatory affairs expert Ana Criado, can offer invaluable assistance in effectively navigating these regulations. bioaccess® specializes in providing customized solutions that align with the regulatory body's requirements, facilitating a smoother approval pathway for Medtech startups.
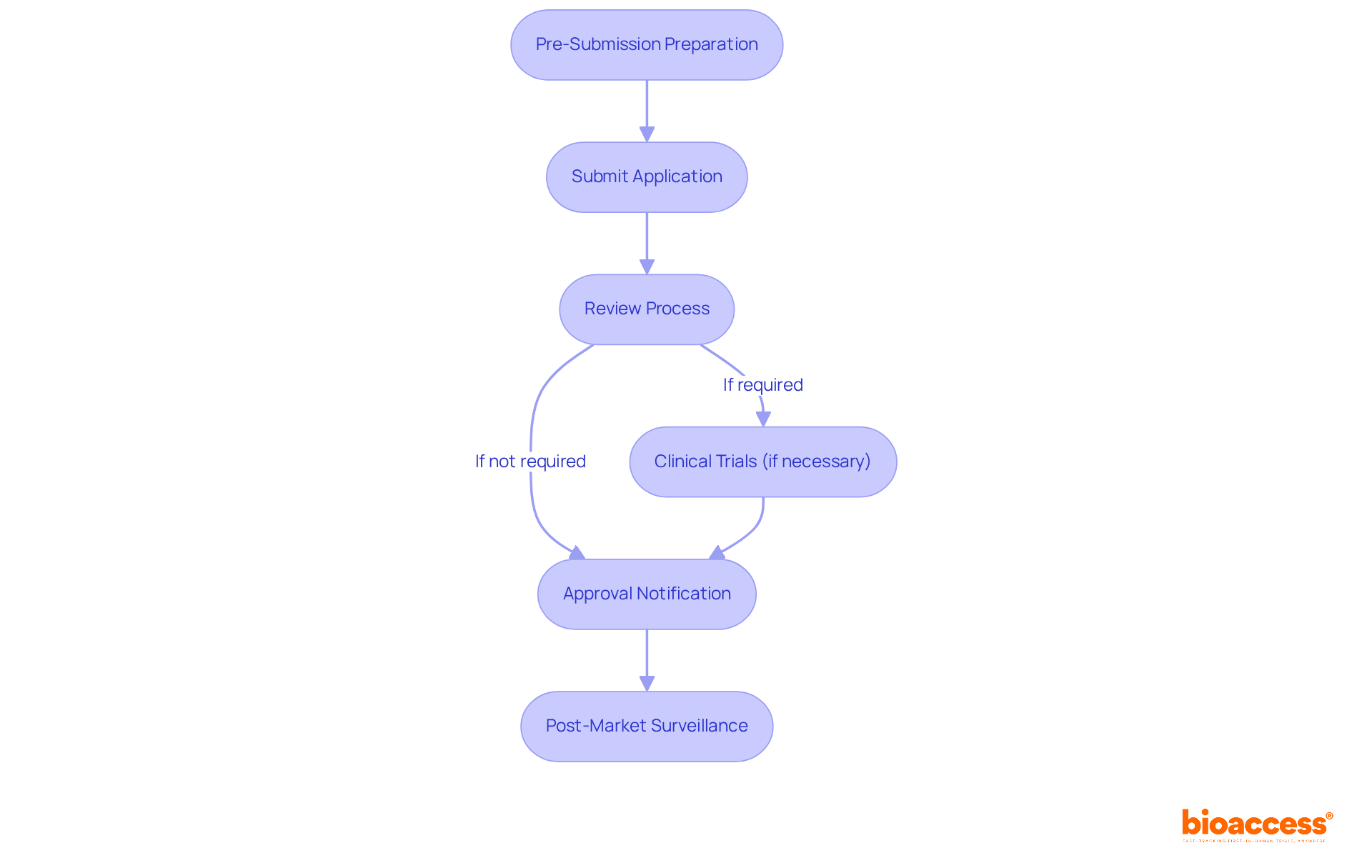
Pre-Submission Preparation: Begin by gathering essential documentation, including product specifications, clinical data, and manufacturing details. It is crucial to categorize your device precisely according to regulatory standards, ensuring a seamless application process. Bioaccess offers comprehensive services, including feasibility studies and the selection of research sites and principal investigators (PIs), to guarantee thorough and compliant preparation.
Submit Application: Complete the application form and send it along with the necessary documents. Adhering to specific submission guidelines is vital to prevent any delays in processing. Our team can provide review and feedback on study documents to ensure compliance with country requirements.
Review Process: The regulatory authority will conduct a comprehensive review of your application. Be prepared to address any queries or requests for additional information that may arise during this phase. Typically, application reviews take around 90 days, which is crucial for organizing your project schedule. Bioaccess can assist in managing this procedure effectively.
Clinical Trials (if necessary): If your product requires clinical trials, ensure they adhere to relevant regulations. Include the trial results in your application to support your submission. Our services encompass trial setup, start-up, and authorization processes, including the acquisition of necessary import permits and the nationalization of investigational devices, along with thorough reporting on study status and adverse events.
Approval Notification: Upon acceptance, you will receive a notification from the regulatory body detailing your registration number and any conditions associated with the endorsement. Bioaccess will help you navigate any post-approval requirements.
Post-Market Surveillance: Following approval, it is essential to maintain compliance with regulatory standards through ongoing post-market surveillance and the reporting of any adverse events. This process is regulated by specific authorities that ensure the ongoing safety and effectiveness of medical products in the market. Our project management and monitoring services will support you in this critical phase.
The online medical equipment sales market in China has undergone remarkable growth, with the number of distributors soaring from 8,717 in 2018 to over 360,000 today. This shift underscores the significance of comprehending the evolving regulatory framework, especially regarding the Chinese NMPA, as the count of third-party platform companies for online medical device sales has increased from 77 to 851. Effective navigation of the regulatory endorsement procedure is essential for capitalizing on this emerging market.
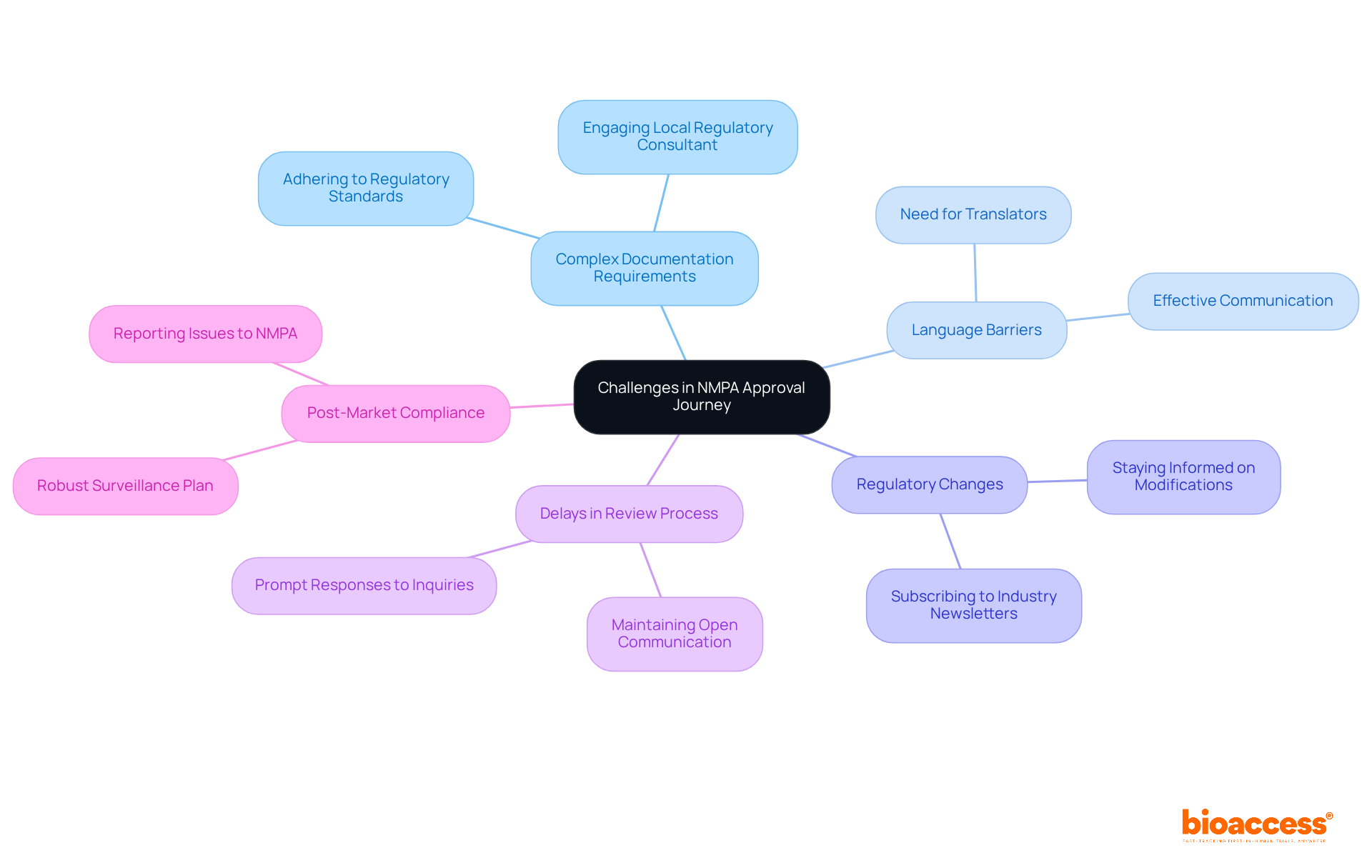
Navigating the NMPA approval process presents several challenges that require careful consideration:
Complex Documentation Requirements: It is essential to ensure that all documents are complete and adhere to regulatory standards. Engaging a local regulatory consultant can significantly assist in this complex Chinese NMPA process.
Language Barriers: For those not fluent in Mandarin, enlisting the help of a translator or local expert is advisable to facilitate effective communication and documentation.
Staying informed about modifications in regulatory guidelines, particularly those related to Chinese NMPA, is crucial, as these changes can impact your application. Regularly visiting the relevant regulatory website or subscribing to industry newsletters will keep you updated.
Delays in Review Process: Be prepared for potential delays in the review process. Maintaining open communication with the Chinese NMPA and responding promptly to inquiries will help keep your application on track.
Post-Market Compliance: After approval, it is vital to have a robust post-market surveillance plan in place. This plan should monitor the performance of your device and ensure any issues are reported to the Chinese NMPA promptly.
Navigating the NMPA approval process is a critical endeavor for companies aiming to enter the Chinese medical device market. Understanding the intricacies of the NMPA's regulatory framework is essential for ensuring that medical devices meet the necessary safety and efficacy standards. By familiarizing oneself with the step-by-step approval process—including pre-submission preparation, application submission, and post-market surveillance—stakeholders can significantly enhance their chances of successful market entry.
Key insights from the article highlight the importance of:
Engaging with experts, such as contract research organizations, can provide invaluable support throughout this complex process. Additionally, awareness of potential challenges—ranging from language barriers to evolving regulatory guidelines—can help companies proactively address obstacles that may arise during their approval journey.
Ultimately, the significance of mastering the NMPA approval process cannot be overstated. As the Chinese market for medical devices continues to expand, staying informed and prepared will be crucial for Medtech startups looking to capitalize on this growing opportunity. By taking the necessary steps to navigate the regulatory landscape effectively, companies can not only ensure compliance but also position themselves for long-term success in the competitive Chinese market.
What is the NMPA and what is its role in China?
The NMPA, or National Medical Products Administration, is China's regulatory authority responsible for overseeing the authorization and regulation of medical devices. It ensures that medical instruments meet stringent safety and effectiveness standards before they can be sold in the Chinese market.
Why is understanding the NMPA important for companies?
Understanding the NMPA is essential for any company, particularly Medtech startups, seeking to enter the Chinese market. Familiarity with the NMPA's guidelines and the classification of medical devices is crucial for navigating the regulatory landscape and obtaining necessary approvals.
What is the first step in the approval process with the NMPA?
The first step in the approval process is to familiarize yourself with the NMPA's guidelines, including the classification of medical devices and the specific criteria for each category. This foundational knowledge is vital for navigating the complexities of the regulatory landscape.
How can companies effectively navigate NMPA regulations?
Companies can effectively navigate NMPA regulations by collaborating with a leading contract research organization (CRO) such as bioaccess®. These organizations provide customized solutions that align with regulatory requirements, facilitating a smoother approval pathway for Medtech startups.
Who can provide insights on navigating NMPA regulations?
Insights from regulatory affairs experts, such as Ana Criado, can provide invaluable assistance in effectively navigating NMPA regulations and understanding the requirements for medical device approval in China.