


In the evolving landscape of clinical research, the expedited review process serves as a crucial mechanism for accelerating the evaluation of research proposals that present minimal risk to participants. This streamlined approach not only enhances the efficiency of the review timeline but also aligns with regulatory efforts to protect human subjects while advancing medical innovations. By understanding the eligibility criteria, categories of research suitable for expedited review, and best practices for proposal submission, researchers can navigate this process effectively.
This article delves into the essential components of expedited reviews, offering insights into how researchers can optimize their submissions to meet regulatory standards and contribute to the swift advancement of impactful medical research.
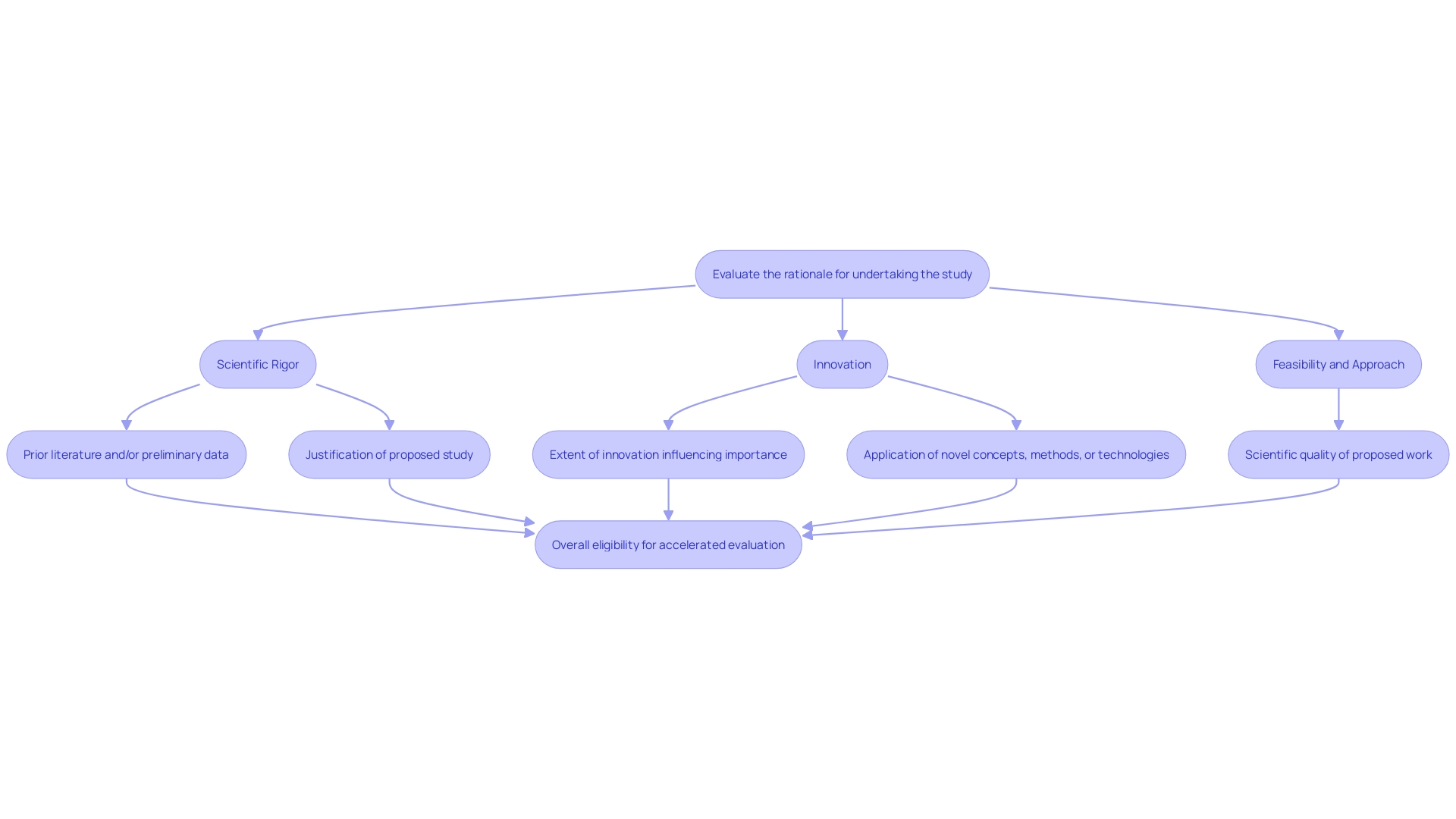
Accelerated evaluation procedures are specifically designed for projects that pose minimal risk to participants, providing an efficient route for initiatives that can greatly influence patient care. This encompasses investigations that make minor adjustments to previously approved research, leverage existing data, or concentrate on groups not considered vulnerable.
To qualify for expedited review, researchers should evaluate several critical factors:
For instance, the Center for Drug Evaluation and Research (CDER) emphasizes that studies supporting designation requests should ideally have been conducted under the existing framework for preparing Investigational New Drug (IND) applications. This not only simplifies the evaluation process but also ensures that the information provided is comprehensive and pertinent. By submitting a designation request that summarizes previously collected data, researchers can facilitate a quicker evaluation while maintaining compliance with regulatory standards.
Furthermore, the FDA is actively working to harmonize human subject protection regulations with the U.S. Department of Health and Human Services' Common Rule. This initiative aims to enhance the efficiency of clinical investigations while safeguarding participant rights, ultimately benefiting the advancement of safe and effective medical products.
Grasping these standards and procedures empowers researchers to maneuver through the accelerated evaluation efficiently, enabling them to concentrate on providing groundbreaking solutions that address urgent medical requirements.
'The accelerated evaluation process can significantly enhance the efficiency of research proposals, particularly for those applications that align with specific categories.'. Research activities that often qualify for expedited review include educational practices, surveys, interviews, and the collection of pre-existing data. Furthermore, studies that employ non-invasive procedures or benign behavioral interventions are also eligible for this expedited pathway.
Grasping these categories is essential, as it can simplify the submission process, minimizing the time required for approvals and enabling faster progress in findings. 'This is particularly relevant in the context of qualitative analysis, where methods like focus groups and in-depth interviews play an essential role in gathering nuanced information about consumer attitudes and behaviors.'. According to the FDA, these qualitative tools allow for a richer understanding of participant motivations and beliefs compared to traditional quantitative methods. Focus groups, for instance, encourage engaging conversations that can uncover information useful for enhancing variables and measures in quantitative research. Additionally, individual interviews can provide a deeper exploration of participants' thoughts and emotions, further enhancing the quality of the findings.
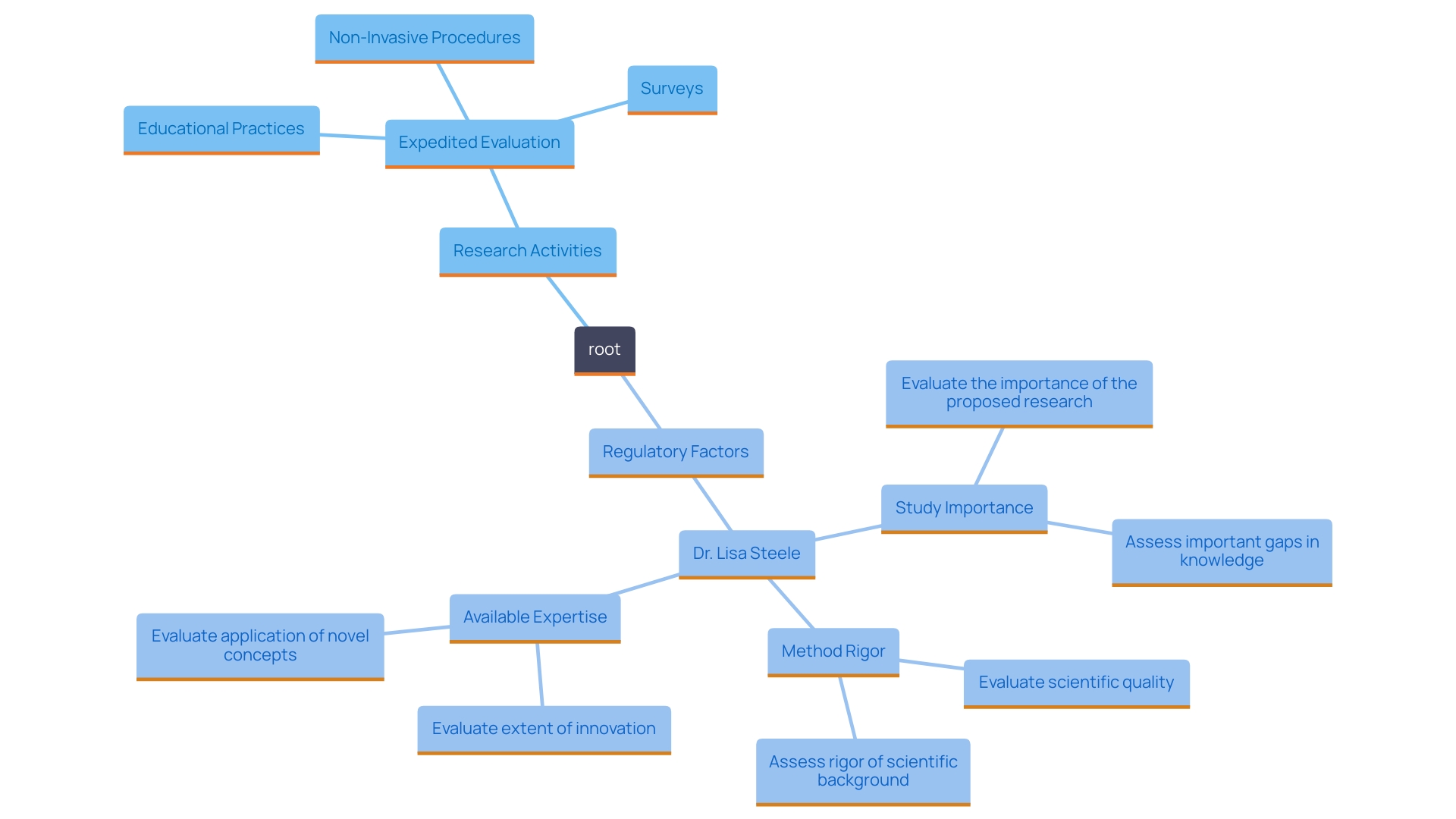
As the landscape of clinical investigation evolves, familiarity with expedited processes not only aids in compliance with regulatory standards but also aligns with emerging review frameworks that emphasize the importance, rigor, and feasibility of proposed studies. Dr. Lisa Steele emphasizes this change, pointing out that regulatory factors are now grouped into three main areas: the importance of the study, the rigor and feasibility of the method, and the expertise and resources available to support the investigation. This renewed focus aims to ensure that impactful projects receive the necessary attention and support, thereby fostering a more efficient and effective investigation environment.
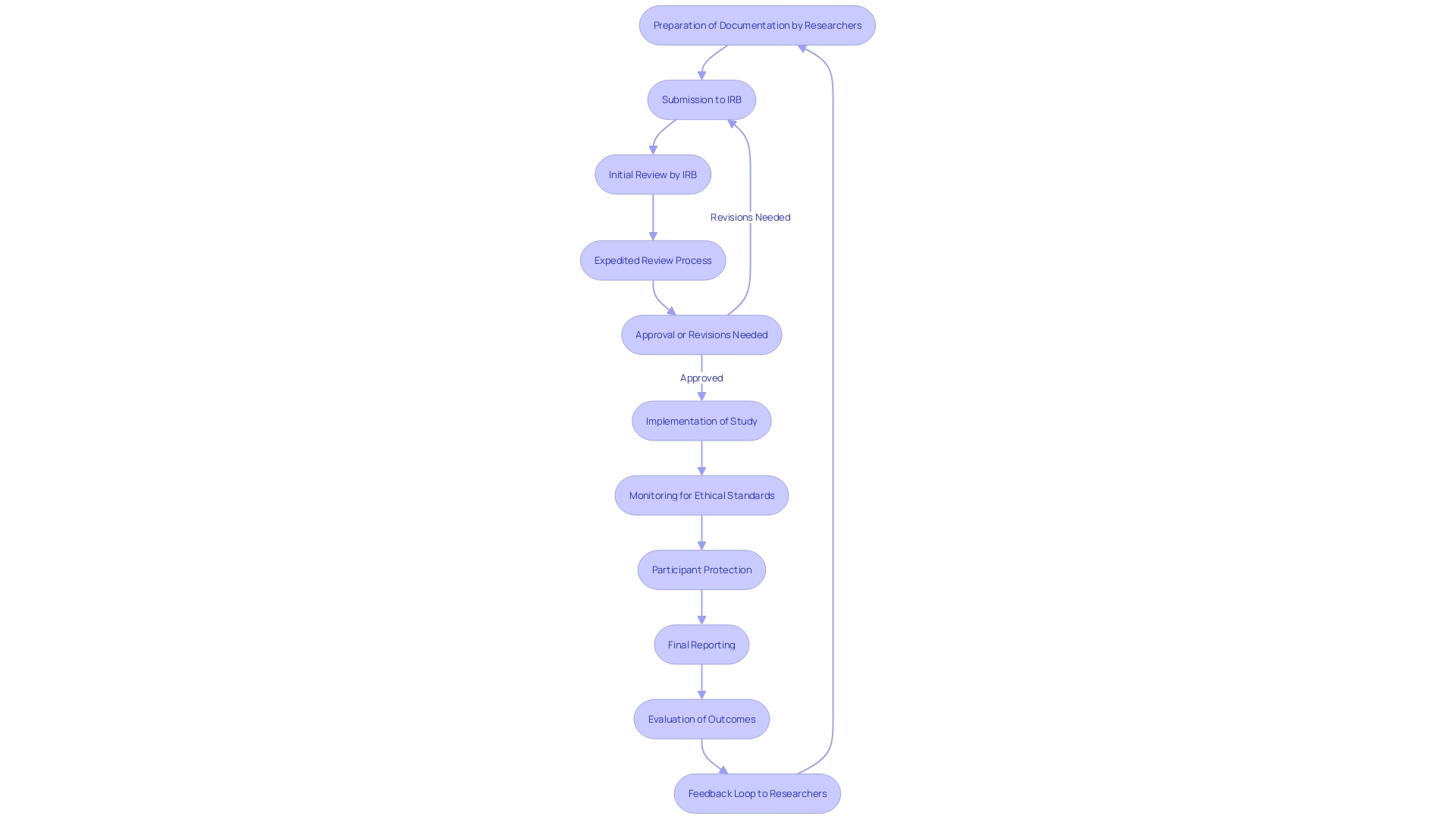
The accelerated assessment method is intended to improve the effectiveness of evaluating research proposals, greatly simplifying the conventional approach. 'In contrast to comprehensive assessments, which generally need a greater quantity of evaluators, expedited evaluations can be performed by one or more members of the Institutional Review Board (IRB).'. This method not only accelerates the evaluation timeline but also ensures adherence to ethical standards and the protection of participants.
Critical to this process is the preparation of comprehensive documentation by researchers. Such documentation aids in facilitating the expedited review, ensuring that the IRB has the necessary information to make informed decisions swiftly. According to the FDA, efficient and well-designed clinical research is vital for advancing the development of safe and effective medical products. This commitment is underscored by the agency's ongoing efforts to harmonize human subject protection regulations with the U.S. Department of Health and Human Services (HHS) Common Rule.
Additionally, the FDA's initiatives highlight that well-organized research and trustworthy information are crucial for informed decision-making concerning the advantages and dangers of medical products. As the landscape of drug development evolves, maintaining rapid evaluation processes will be crucial in balancing the need for timely access to new therapies while safeguarding participant rights and ensuring that ethical standards are upheld.
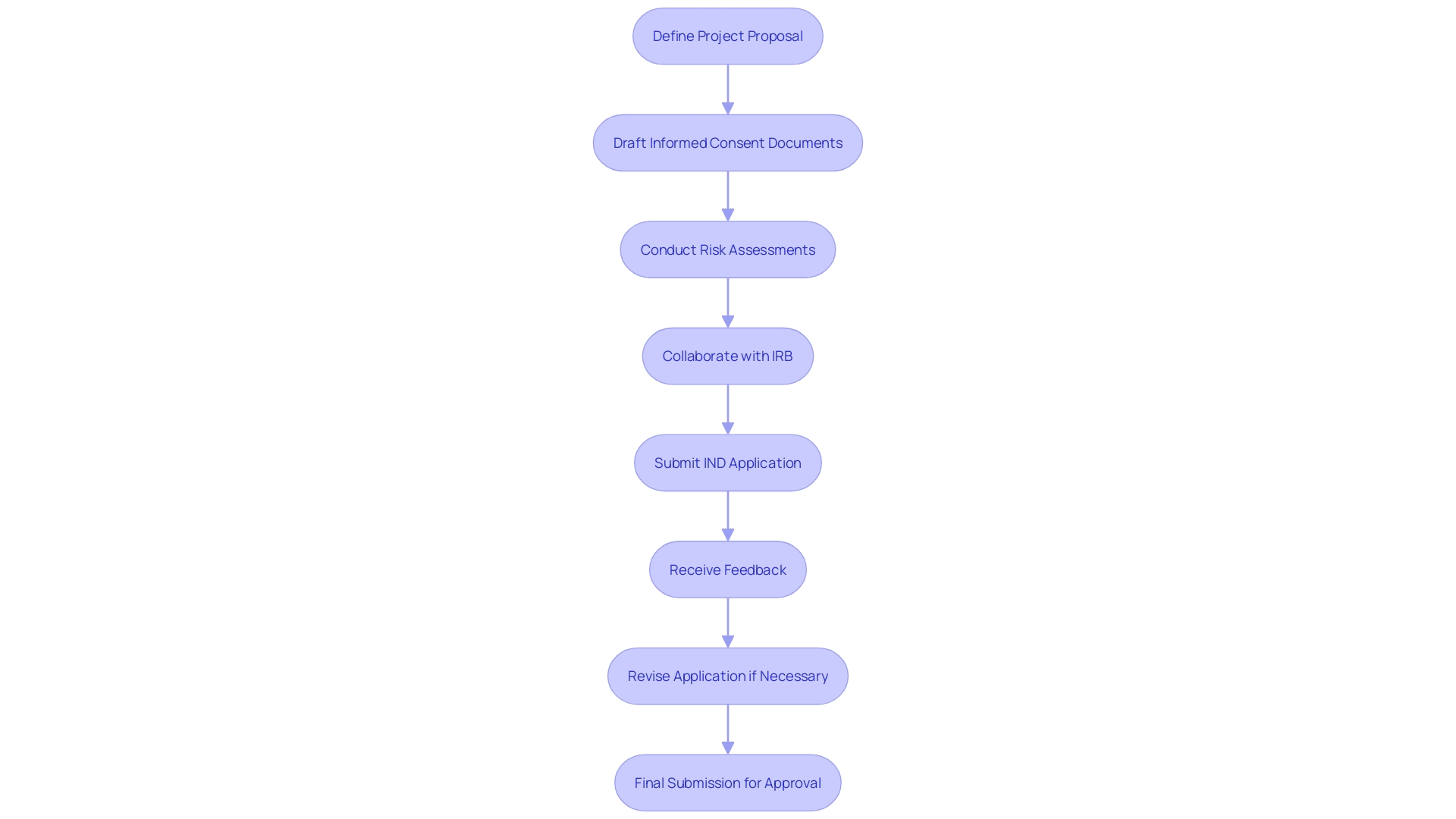
To enhance the likelihood of approval during expedited evaluation for IND applications, researchers must implement best practices that clearly articulate their study objectives and methodologies. A well-defined project proposal should begin with a concise title and an abstract summarizing the inquiry question, objectives, and anticipated outcomes. This foundational clarity not only helps streamline the review but also sets the stage for a transparent informed consent procedure, which is crucial for ethical compliance.
Informed consent documents should present key information in a manner that facilitates participant understanding. This includes clearly outlining the purpose of the investigation, potential risks and benefits, and the study's procedures and duration. The National Academy of Sciences emphasizes the importance of clear communication, stating that informed consent should effectively convey why someone might choose to participate or decline.
Thorough documentation is essential in this context. Researchers are advised to include risk assessments and a robust justification for seeking expedited status. Engaging with the Institutional Review Board (IRB) early in the process can help address any concerns and refine the proposal before formal submission. Misunderstanding terms such as 'delayed start' and 'delayed onset' can adversely affect application evaluations, underscoring the need for precision in research planning and documentation.
By ensuring comprehensive and meticulous submission materials, researchers can significantly enhance their chances of a favorable expedited review outcome.
The expedited review process is essential for advancing clinical research while safeguarding participant rights. By focusing on studies that present minimal risk, this approach streamlines the evaluation of research proposals, enabling quicker access to vital medical innovations. Understanding the eligibility criteria, including scientific rigor, innovation, and feasibility, is paramount for researchers aiming to optimize their submissions.
Categories of research eligible for expedited review encompass a wide range of activities, from educational practices to the collection of pre-existing data. Familiarity with these categories not only facilitates a more efficient submission process but also supports the development of qualitative research methodologies that yield richer insights. The emphasis on significant, rigorous, and feasible research ensures that impactful projects receive the attention they deserve, thereby enhancing the overall research landscape.
Best practices for submitting proposals play a critical role in the expedited review process. Clear articulation of study objectives, thorough documentation, and effective informed consent communication are vital components that can significantly influence the outcome of the review. Engaging with the Institutional Review Board early in the process can further refine proposals and address potential concerns, ultimately leading to a more favorable evaluation.
In summary, navigating the expedited review process requires a comprehensive understanding of its components and best practices. By adhering to these guidelines, researchers can contribute to the efficient advancement of medical research, ensuring that innovations reach those in need while maintaining the highest ethical standards.
What are accelerated evaluation procedures?
Accelerated evaluation procedures are designed for research projects that pose minimal risk to participants. They provide an efficient route for initiatives that can significantly influence patient care, often involving minor adjustments to previously approved studies, utilizing existing data, or focusing on non-vulnerable groups.
What factors are critical for qualifying for expedited review?
Researchers must evaluate the following factors: Scientific Rigor, Innovation, and Feasibility and Approach.
How does the Center for Drug Evaluation and Research (CDER) support expedited review?
CDER emphasizes that studies supporting designation requests should ideally align with the existing framework for Investigational New Drug (IND) applications. This helps simplify the evaluation and ensures comprehensive and relevant information is provided.
What types of studies often qualify for expedited review?
Research activities that typically qualify include educational practices, surveys, interviews, collection of pre-existing data, non-invasive procedures, and benign behavioral interventions.
Why is familiarity with expedited processes important for researchers?
Understanding expedited processes aids in compliance with regulatory standards and aligns with emerging frameworks that emphasize the importance, rigor, and feasibility of studies, thus ensuring impactful projects receive necessary attention.
How does the accelerated assessment method differ from comprehensive assessments?
The accelerated assessment method allows for quicker evaluations, often conducted by one or more members of the Institutional Review Board (IRB), reducing the number of evaluators needed compared to comprehensive assessments.
What role does documentation play in the expedited evaluation process?
Comprehensive documentation is critical as it facilitates the expedited review process, ensuring that the IRB has the necessary information to make informed decisions quickly.
How can researchers enhance the likelihood of approval during expedited evaluation?
Researchers should implement best practices by clearly articulating study objectives and methodologies. This includes providing a concise title, an abstract, informed consent documents, and a robust justification for seeking expedited status.
What is the importance of informed consent in expedited evaluations?
Informed consent documents must clearly outline the purpose, potential risks and benefits, and procedures of the study to ensure participant understanding and compliance with ethical standards.
What should researchers avoid to ensure a successful application?
Researchers should avoid misunderstandings of terms like 'delayed start' and 'delayed onset,' as these can negatively impact application evaluations. Precision in research planning and documentation is essential.