


The landscape of medical devices is intricately tied to the regulatory frameworks that govern their development, testing, and market approval. Understanding the classification of medical devices is essential for navigating the complexities associated with safety and efficacy assessments. The regulatory environment, shaped by entities such as the FDA and the EU Medical Device Regulation, delineates clear pathways for clinical trials, each with its own set of requirements and challenges.
As medical device leaders navigate these regulations, they face critical hurdles, including participant recruitment, compliance with evolving standards, and the intricacies of multi-regional trials. This article delves into the classification of medical devices, the structured stages of clinical trials, the regulatory pathways that guide their approval, and the challenges inherent in this vital sector. By examining these elements, a comprehensive understanding of the medical device landscape emerges, highlighting the importance of rigorous oversight and ethical considerations in ensuring patient safety and effective healthcare technologies.
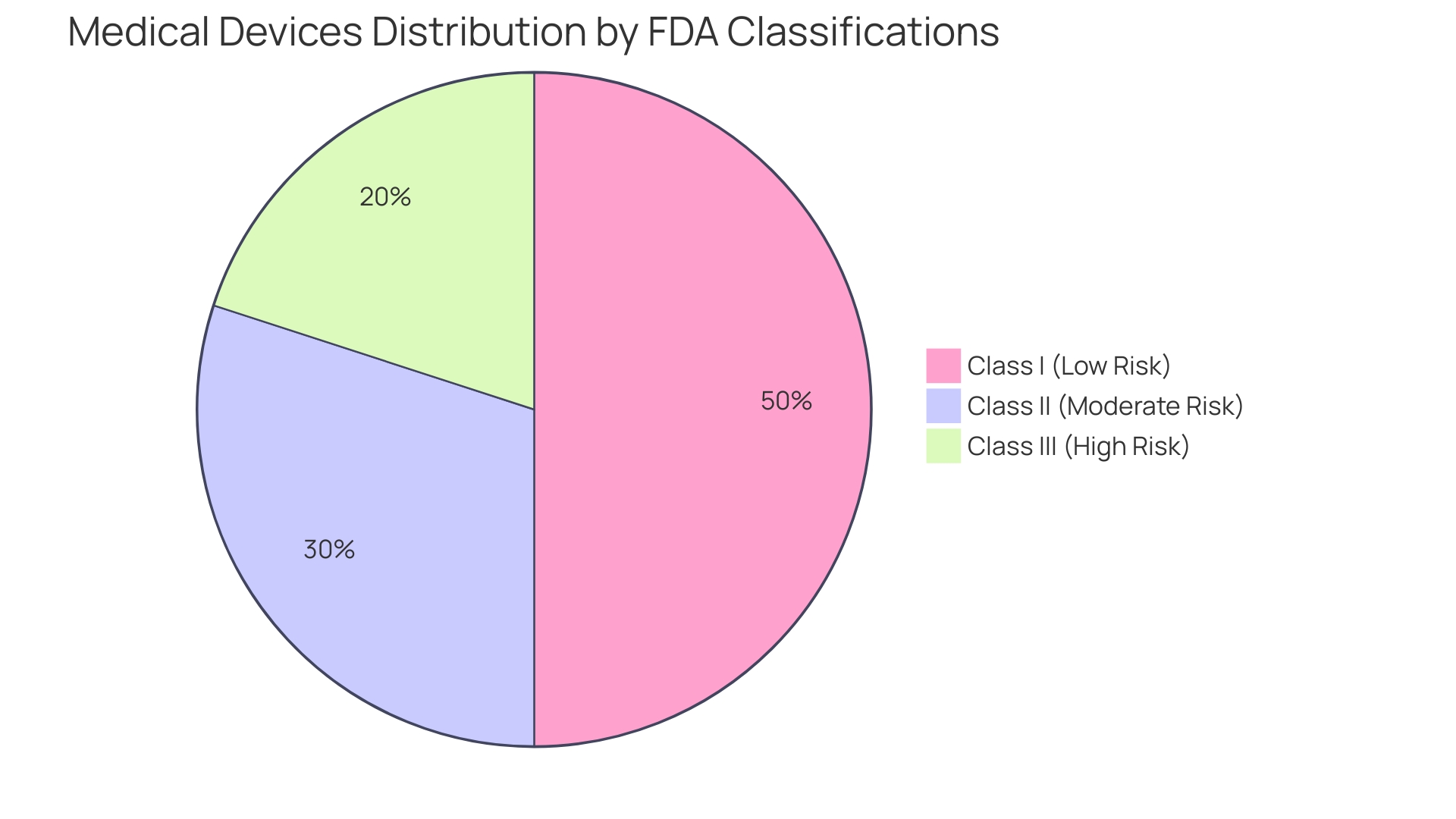
'Medical instruments are systematically classified based on their intended application and the associated risk levels, which play a vital role in determining oversight pathways and trial designs.'. The FDA classifies these devices into three main classes:
Comprehending these classifications is not just an academic task; it has practical consequences for the development and market approval procedures of medical products. For example, a recent study emphasized that 47% of medical equipment leaders recognized ensuring adherence to governing organizations as a top priority, reflecting the difficulties presented by changing laws and heightened examination.
Moreover, the complexities of the regulatory landscape are underscored by the need for comprehensive pre-market research and post-market surveillance. The FDA mandates that manufacturers submit a Summary of Safety and Clinical Performance (SSCP) for Class III products after they hit the market, which is crucial for monitoring long-term safety and efficacy. This requirement stems from the acknowledgment that over 1.7 million injuries and 83,000 fatalities in the United States over a decade were potentially associated with medical equipment, as indicated in a 2018 study of FDA data.
Furthermore, while the FDA’s 510(k) clearance process permits certain products to be fast-tracked without clinical trials, this has raised ethical and safety concerns. The documentary The Bleeding Edge revealed that many products cleared under this process led to significant patient injuries, prompting calls for more stringent oversight.
Ultimately, navigating the regulatory landscape requires a nuanced understanding of these classifications and the ethical considerations surrounding the development and deployment of medical technologies. As the landscape of digital health and MedTech continues to evolve, ongoing dialogue about the ethical implications and governance of these tools will be essential.
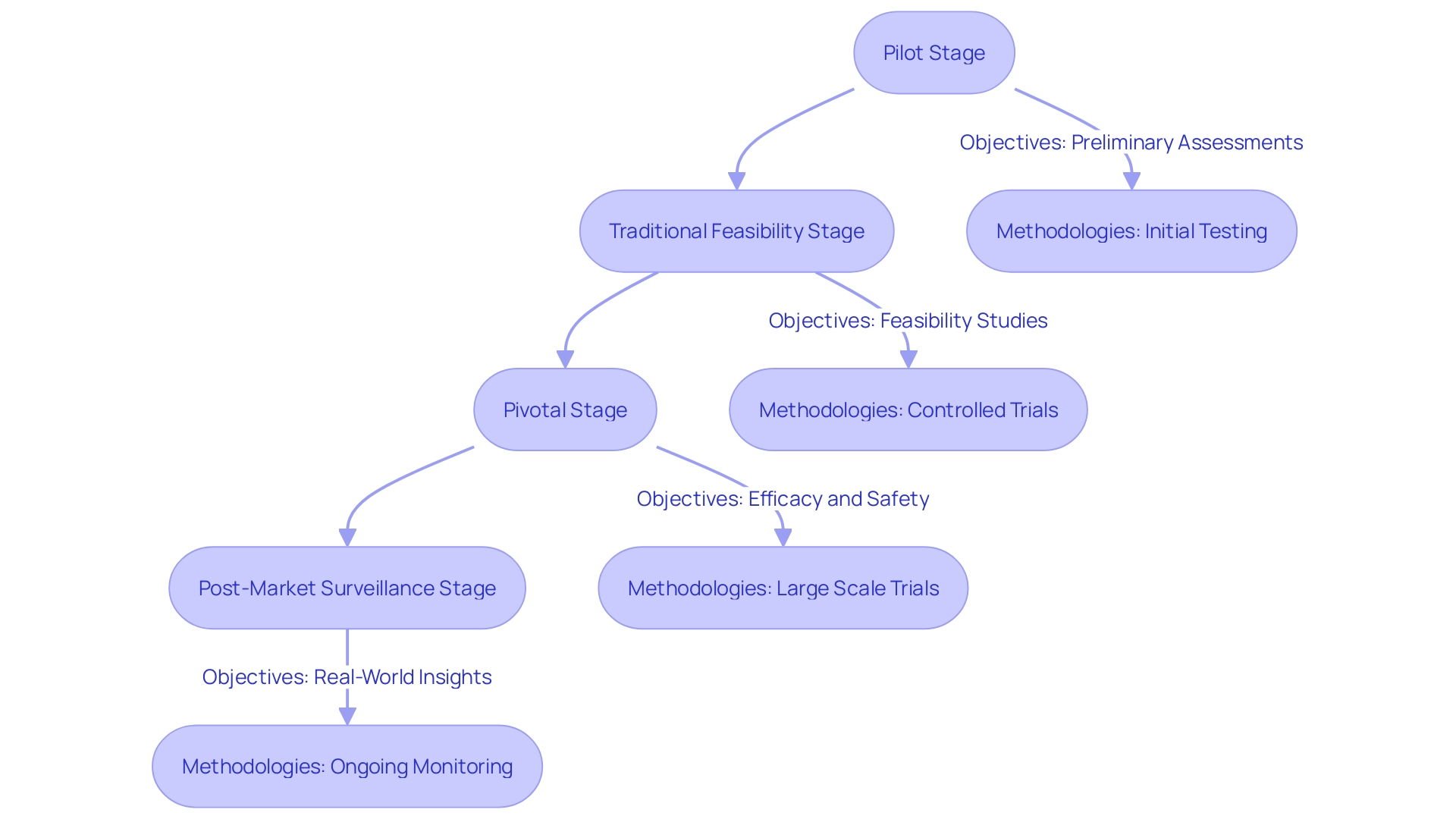
Clinical trials for medical instruments are meticulously structured to ensure thorough evaluation of safety and efficacy, comprising various distinct stages: the pilot stage, the traditional feasibility stage, the pivotal stage, and the post-market surveillance stage. Each of these phases serves specific objectives and employs unique methodologies, progressively validating the apparatus through systematic testing and analysis.
The pilot stage is essential for preliminary feasibility evaluations and often entails restricted user engagement to assess the practicality of the system. During the traditional feasibility stage, more comprehensive testing is conducted, allowing for adjustments based on preliminary feedback. This phase seeks to enhance the apparatus before it reaches the crucial point, where stringent evaluations are conducted, directly engaging a larger group of participants. At this juncture, the emphasis is on collecting robust medical data to support the device's regulatory submission.
Post-market surveillance is an often underutilized but essential phase, providing insights derived from real-world usage. Data obtained during this stage is vital for ongoing assessment and can reveal safety concerns that may not have emerged during earlier trials. As one expert noted, "Post-market surveillance data plays a crucial yet often underutilized role in MedTech evaluations.". This data, derived from both similar and the company's own products, needs to be analyzed with the same rigor as the primary data."
Additionally, the oversight framework requires that a well-organized Clinical Evaluation Report (CER) is crucial for proving a device’s safety and performance. The CER must be based on a thorough evaluation of medical data gathered from diverse sources, including independent studies.
'With oversight scrutiny on the rise—highlighted by the fact that more than 1.7 million injuries and 83,000 deaths in the U.S. over a decade were potentially linked to medical devices—adhering to these structured phases is not just a compliance requirement but a necessity for ensuring patient safety and achieving market success.'.
Maneuvering through the compliance environment for research studies is essential for the effective advancement and market introduction of medical instruments. In the United States, the Investigational Device Exemption (IDE) is a key component that permits the clinical testing of instruments not yet cleared for marketing by the FDA. This exemption allows researchers to gather vital safety and effectiveness data, which is indispensable for obtaining subsequent market approval. As emphasized by industry experts, familiarizing oneself with the FDA's regulations and guidance documents is essential before initiating a study, ensuring all necessary protocols are followed.
In Europe, the oversight environment is governed by the EU Medical Device Regulation (EU MDR). This framework mandates compliance for all new medical instruments entering the market, with existing products facing a transition period for recertification. A recent report indicated that 47% of medical device leaders identified ensuring adherence to governing bodies as a top priority, underscoring the increasing complexity and scrutiny within the sector.
Moreover, as the landscape evolves, the UK Medicines and Healthcare products Regulatory Agency (MHRA) has announced new regulations aimed at enhancing patient safety and ensuring timely access to innovative medical technologies. These regulations will focus on transformative technologies, including healthcare AI and diagnostics, and are expected to be fully implemented by 2025.
The requirement for comprehensive examination and clear presentation of medical data is more vital than ever. Numerous experts face difficulties in coordinating data among different documents, resulting in discrepancies that can impede approval procedures. Employing post-market surveillance information efficiently is frequently neglected but can offer important insights that enhance evaluations in healthcare. As the demand for digital health solutions rises, particularly in the Software as a Medical Device (SaMD) sector, understanding the nuanced regulatory requirements will be vital for success in this rapidly growing market.
Carrying out research studies for medical devices is filled with unique obstacles that can greatly affect the success of these efforts. One major hurdle is the recruitment of suitable participants, which often requires navigating complex demographics and varying healthcare access across regions. For instance, the rise of telemedicine has opened avenues to reach low-income and underserved populations, enhancing participant diversity and representation.
Patient safety is paramount throughout the trial process. As articulated in the European Union Medical Device Regulation (EU MDR), investigations must protect the rights, safety, dignity, and well-being of participants, emphasizing the need for rigorous protocols and oversight. This governing structure not only protects participants but also guarantees that data produced is scientifically sound and trustworthy.
'Adherence to legal standards is another critical aspect of clinical trials.'. The evolving landscape of regulatory requirements necessitates that firms adopt strategic planning and robust methodologies to thrive amidst these changes. For example, the FDA plays a crucial role in ensuring the safety and effectiveness of medical devices, necessitating comprehensive documentation and adherence to guidelines for successful market entry.
Furthermore, variations in international regulations can complicate multi-regional studies. A significant observation from industry experts is that operational complexities often arise from changes in project dynamics, such as job changes within test locations and the allocation of resources. This complexity highlights the significance of maintaining clear communication and detailed planning to align all stakeholders with project objectives.
In summary, overcoming the obstacles related to medical research for healthcare tools necessitates a comprehensive strategy that emphasizes participant safety, guarantees adherence, and adjusts to the evolving regulatory environment. With approximately 10-15% of successful 510(k) submissions for Class II devices relying on clinical trial data, effective management of these trials is essential for successful outcomes.
The classification of medical devices is crucial for determining regulatory pathways and trial designs. By categorizing devices into Class I, II, and III based on risk, stakeholders can better navigate compliance challenges and enhance patient safety, especially given the concerning statistics on device-related injuries and fatalities.
Clinical trials follow structured stages—from pilot assessments to post-market surveillance—to ensure thorough evaluation of safety and efficacy. Each phase collects and analyzes clinical data systematically, reinforcing trust in medical technologies and benefiting patient care.
Navigating regulatory pathways is essential for market entry. The Investigational Device Exemption (IDE) in the U.S. and the EU Medical Device Regulation (EU MDR) provide vital guidelines that manufacturers must follow. As these regulations evolve, medical device leaders must stay informed and adaptable to promote innovation while ensuring compliance.
Conducting clinical trials also presents challenges, such as participant recruitment and compliance with varying international standards. Strategic planning is vital for overcoming these hurdles and achieving successful outcomes. In summary, a deep understanding of the regulatory landscape, combined with a commitment to ethical practices, is essential for advancing medical technologies while prioritizing patient safety and well-being.
How are medical instruments classified?
Medical instruments are classified by the FDA into three classes based on their intended application and associated risk levels: Class I (low risk, minimal regulatory control), Class II (moderate risk, more stringent oversight), and Class III (high risk, rigorous evaluation required).
What are the implications of these classifications?
Understanding these classifications is crucial for the development and market approval of medical products, as they determine the regulatory pathways and trial designs necessary for compliance.
What is the FDA's 510(k) clearance process?
The 510(k) clearance process allows certain medical devices to be fast-tracked for market entry without extensive clinical trials, raising ethical concerns due to significant patient injuries associated with some cleared products.
What are the stages of clinical trials for medical instruments?
Clinical trials comprise several stages: Pilot Stage (preliminary evaluations), Traditional Feasibility Stage (comprehensive testing), Pivotal Stage (stringent evaluations with larger groups), and Post-Market Surveillance Stage (ongoing safety assessment).
Why is post-market surveillance important?
Post-market surveillance is essential for identifying safety concerns that may not have emerged during earlier trials, providing insights for ongoing assessment of medical devices.
What is the Investigational Device Exemption (IDE)?
The Investigational Device Exemption (IDE) allows clinical testing of medical instruments not yet cleared for marketing by the FDA, critical for gathering safety and effectiveness data for future approval.
How does the regulatory landscape differ between the U.S. and Europe?
In Europe, the EU Medical Device Regulation (EU MDR) governs compliance for new medical instruments, while the U.S. has its own regulatory framework, including FDA oversight.
What are some challenges in conducting research studies for medical devices?
Challenges include recruiting suitable participants, ensuring patient safety, and navigating evolving regulatory requirements while maintaining clear communication among stakeholders.
How critical is adherence to legal standards in clinical trials?
Adherence to legal standards is vital for ensuring safety, effectiveness, and scientific integrity in clinical trials, requiring comprehensive documentation and compliance with regulatory guidelines.
What is the significance of clinical trial data for Class II devices?
Approximately 10-15% of successful 510(k) submissions for Class II devices rely on clinical trial data, making effective management of these trials crucial for successful market outcomes.