

This article focuses on optimizing clinic operations for effective clinical research management. It defines roles, ensures compliance, implements recruitment strategies, and utilizes regional advantages. A clear organizational structure enhances efficiency and accountability, while adherence to regulations improves trial outcomes. Additionally, strategic recruitment and location selection can significantly accelerate research timelines and reduce costs. By understanding these elements, clinics can better navigate the complexities of clinical research and achieve more favorable results.
In the complex landscape of clinical research, optimizing clinic operations transcends mere administration; it stands as a pivotal factor determining the success of a trial. By clearly defining roles, ensuring compliance with regulations, and implementing effective recruitment strategies, organizations can significantly enhance their operational efficiency and trial outcomes.
However, with the ever-evolving regulatory environment and the challenges of attracting top talent, how can research teams navigate these complexities to achieve their goals? This article delves into best practices for streamlining clinic operations, offering insights and actionable strategies to empower clinical research management.
Establishing a clear organizational structure in clinic operations is essential for effectively delineating roles and responsibilities. This framework should outline key roles such as Clinical Research Associates (CRAs), Project Managers, and Regulatory Affairs Specialists, each with distinct responsibilities that contribute to the study's success. For instance, CRAs are primarily responsible for site management and ensuring data integrity, while Project Managers oversee the overall project timeline and resource allocation. A clearly outlined framework not only boosts team efficiency but also enhances outcomes and accelerates patient enrollment. Furthermore, bioaccess offers a comprehensive process that encompasses setup, initiation, and authorization, along with reporting, which are vital for successful research studies.
Research leaders emphasize that a successful organizational structure enhances clinic operations by promoting teamwork and accountability, ultimately resulting in more efficient trials. By clearly defining roles, teams can reduce confusion and enhance processes, which is critical in the fast-paced environment of medical research.
Actionable Tip: Develop a detailed organizational chart that specifies each role and its responsibilities. Consistently examine and refresh this chart to adapt to any changes in group dynamics or project scope, ensuring that all members are aligned and informed.
To ensure compliance with regulations and ethical standards, research groups must remain vigilant regarding the latest guidelines from regulatory authorities such as the FDA and EMA. This necessitates a comprehensive understanding of Good Clinical Practice (GCP), which is essential for safeguarding patient safety and upholding the integrity of research trials. Regular training sessions are vital for keeping the team informed about compliance requirements and ethical considerations, as GCP guidelines evolve to reflect contemporary research environments.
Establishing a robust compliance monitoring system can proactively identify potential issues, thereby enhancing the overall quality of clinical research. Notably, adherence to GCP has been shown to significantly improve trial outcomes, with studies indicating that compliance can lead to faster regulatory approvals and increased participant trust.
Actionable Tip: Develop a comprehensive compliance checklist that encompasses all regulatory requirements and ethical standards pertinent to your studies. Utilize this checklist during audits and reviews to ensure consistent adherence and foster a culture of compliance within your group.
To develop a high-performing research group, organizations must implement focused hiring strategies that emphasize candidates with the necessary experience and skills. Effective methods include:
Notably, leveraging bioaccess™ extensive presence in Colombia has proven successful in enhancing outreach and engagement with potential candidates. Furthermore, cultivating a positive organizational culture that fosters collaboration and professional growth is crucial for retaining top talent. By implementing mentorship programs and offering continuous education opportunities, organizations can significantly enhance team capabilities.
Statistics reveal that 37% of study sites encounter challenges related to under-enrollment, underscoring the necessity for effective hiring strategies. A robust hiring strategy should clearly delineate the skills and qualifications needed for each role while actively promoting the organization’s culture and values throughout the selection process. This strategic approach not only attracts exceptional talent but also ensures a united and driven research group, ultimately resulting in more successful studies.
The collaboration between GlobalCare Clinical Trials and bioaccess™ exemplifies how strategic hiring can yield remarkable outcomes. By capitalizing on bioaccess™ significant presence in Colombia, GCCT achieved over a 50% reduction in clinical trial participant enrollment time and a retention rate exceeding 95%. This case illustrates the impact of efficient hiring strategies on operational success.
Actionable Tip: Develop a recruitment plan that outlines the skills and qualifications needed for each role, and actively promote your organization’s culture and values during the hiring process, as demonstrated by the successful partnership between GlobalCare Clinical Trials and bioaccess™.
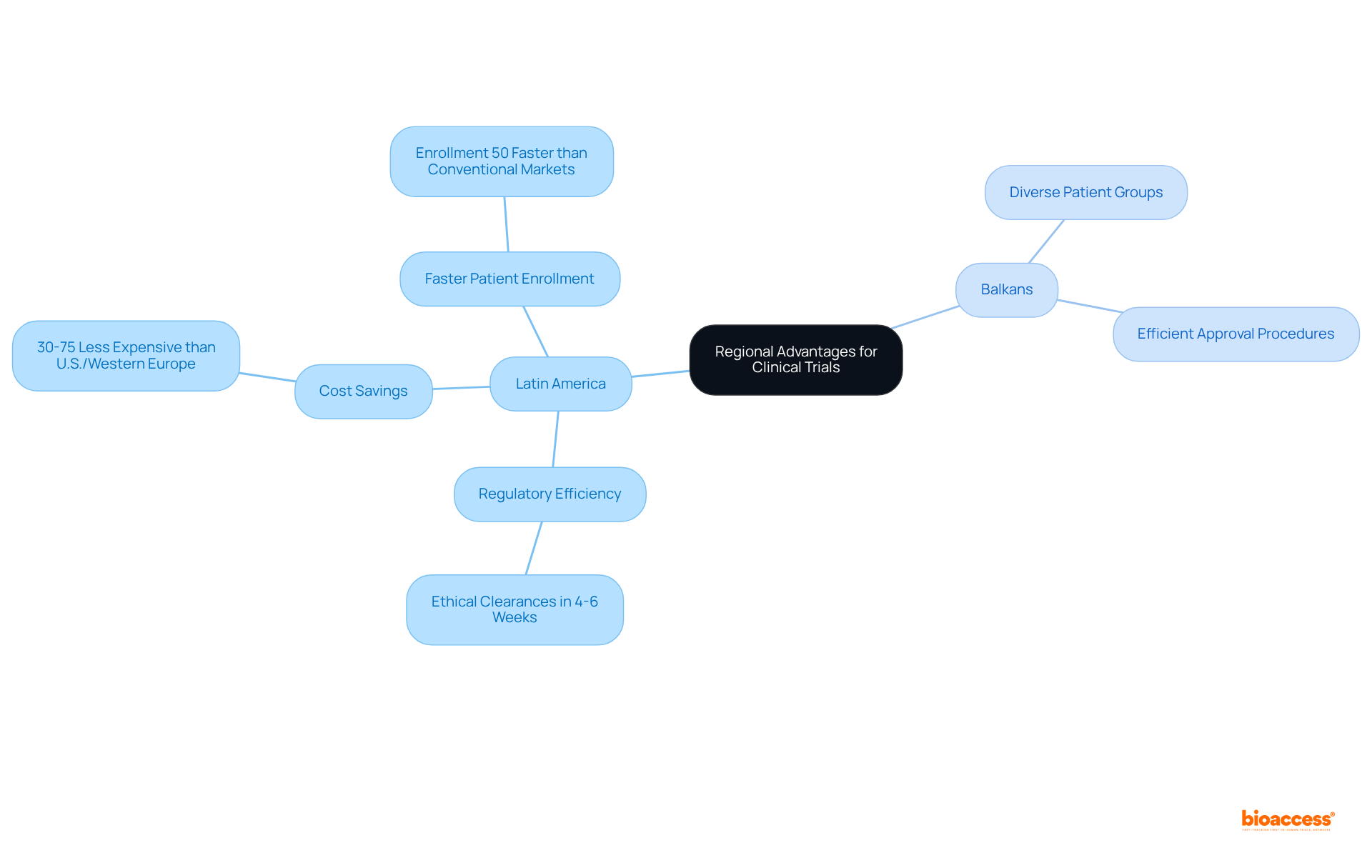
To fully leverage regional advantages, research organizations must conduct comprehensive market analyses that illuminate the unique benefits of each location. Latin America, for example, stands out for its swift regulatory approvals, with ethical clearances typically obtained in just 4-6 weeks. This remarkable efficiency significantly shortens research timelines, facilitating patient enrollment that can be up to 50% faster than in conventional markets. Additionally, conducting tests in Latin America can be 30% to 75% less expensive than in the U.S. or Western Europe, making it a financially appealing option for sponsors. bioaccess® excels in managing a variety of studies that support clinic operations, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Clinical Follow-Up Studies. Their extensive expertise in navigating these processes related to clinic operations encompasses feasibility studies, compliance reviews, and project management. Concurrently, the Balkans offer access to diverse patient groups and efficient approval procedures, enhancing the representativeness of research studies and improving participant acquisition—an alignment with bioaccess's commitment to effective study management.
Strategically selecting test locations based on these factors not only bolsters recruitment efforts but also minimizes operational delays in clinic operations. Establishing robust connections with local regulatory authorities is crucial, as it can streamline approval processes and foster a collaborative environment for clinical studies.
Actionable Tip: Develop a regional strategy document that outlines the specific advantages of each trial location, including expedited enrollment rates and cost efficiencies. This resource should serve as a guide for site selection and clinic operations planning, ensuring that organizations capitalize on the strengths of their chosen regions.
Establishing efficient clinic operations is paramount for successful clinical research management. By clearly defining roles and responsibilities, ensuring compliance with regulations, implementing effective recruitment strategies, and utilizing regional advantages, organizations can enhance their operational effectiveness and improve trial outcomes. This integrated approach not only streamlines processes but also fosters a collaborative environment that is essential for navigating the complexities of clinical research.
Key insights underscore the significance of a well-structured team with defined roles, minimizing confusion and enhancing accountability. Compliance with Good Clinical Practice (GCP) is vital for maintaining patient safety and integrity, while targeted recruitment strategies ensure that skilled professionals contribute to the research efforts. Furthermore, leveraging regional advantages can significantly accelerate trial timelines and reduce costs, making it a strategic imperative for research organizations.
In conclusion, optimizing clinic operations transcends mere efficiency; it is a critical factor influencing the success of clinical trials. By adopting best practices in management, compliance, and recruitment, organizations can position themselves for greater success in the competitive landscape of clinical research. Embracing these strategies will not only enhance operational performance but will ultimately lead to better outcomes for patients and the advancement of medical science.
Why is it important to define roles and responsibilities in clinical operations?
Defining roles and responsibilities is essential for establishing a clear organizational structure, which enhances team efficiency, promotes teamwork and accountability, and ultimately leads to more effective clinical trials.
What are some key roles in clinical operations?
Key roles in clinical operations include Clinical Research Associates (CRAs), Project Managers, and Regulatory Affairs Specialists, each with distinct responsibilities that contribute to the success of the study.
What are the specific responsibilities of Clinical Research Associates (CRAs)?
CRAs are primarily responsible for site management and ensuring data integrity throughout the clinical trial process.
What role do Project Managers play in clinical operations?
Project Managers oversee the overall project timeline and resource allocation, ensuring that the study progresses efficiently.
How can a clearly outlined framework benefit clinical operations?
A clearly outlined framework boosts team efficiency, enhances outcomes, accelerates patient enrollment, and reduces confusion among team members.
What process does bioaccess offer for successful research studies?
Bioaccess offers a comprehensive process that encompasses setup, initiation, authorization, and reporting, which are vital for successful research studies.
How can research leaders improve clinic operations?
Research leaders can improve clinic operations by promoting teamwork and accountability through a successful organizational structure, which results in more efficient trials.
What is an actionable tip for managing roles and responsibilities in clinical operations?
An actionable tip is to develop a detailed organizational chart that specifies each role and its responsibilities, and to consistently examine and refresh this chart to adapt to changes in group dynamics or project scope.