


The article delineates crucial steps for effectively ordering a post-market compliance package in Mexico, underscoring the necessity of adhering to COFEPRIS regulations concerning medical devices. It asserts that:
are vital for upholding compliance and safeguarding patient safety. This approach mitigates risks, including potential penalties or loss of market access, thereby reinforcing the imperative for diligence in this domain.
Navigating the intricate landscape of post-market compliance in Mexico is essential for manufacturers of medical devices. Adherence to the regulations established by COFEPRIS can profoundly influence both product success and patient safety. This guide explores the critical steps for procuring a post-market compliance package in Mexico, providing insights into regulatory requirements and best practices that ensure continued conformity and market access. Given the serious repercussions associated with non-compliance, how can manufacturers effectively protect their interests while upholding the highest standards of safety and efficacy?
Post-market adherence in Mexico is governed by the regulations established by COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios), necessitating continuous monitoring of the reliability and performance of medical devices post-approval. To effectively track adverse events, conduct regular audits, and ensure compliance with reporting requirements, it is essential to order a post-market compliance package Mexico. This vigilance is crucial for maintaining market authorization and safeguarding patient safety. Key components of an effective post-market compliance strategy include:
Statistics indicate that enrollment for clinical studies in Mexico is 50% faster than in traditional markets, showcasing the efficiency of the regulatory environment. Furthermore, the implementation of a technovigilance system, as mandated by NOM-240, is critical for monitoring equipment reliability and performance and is necessary to order post-market compliance package Mexico to ensure prompt reporting of incidents. The technovigilance report serves as a documentation necessity for the initial renewal of COFEPRIS registration, underscoring the importance of ordering post-market compliance package Mexico for meticulous documentation in regulatory efforts. Collaborating with local compliance experts can streamline the approval process and provide insights into the latest COFEPRIS requirements, ultimately enhancing the security and effectiveness of medical products in the market. Additionally, the potential consequences of non-compliance, including penalties and loss of market access, further underscore the urgency of adhering to regulations in this multi-billion dollar healthcare market.
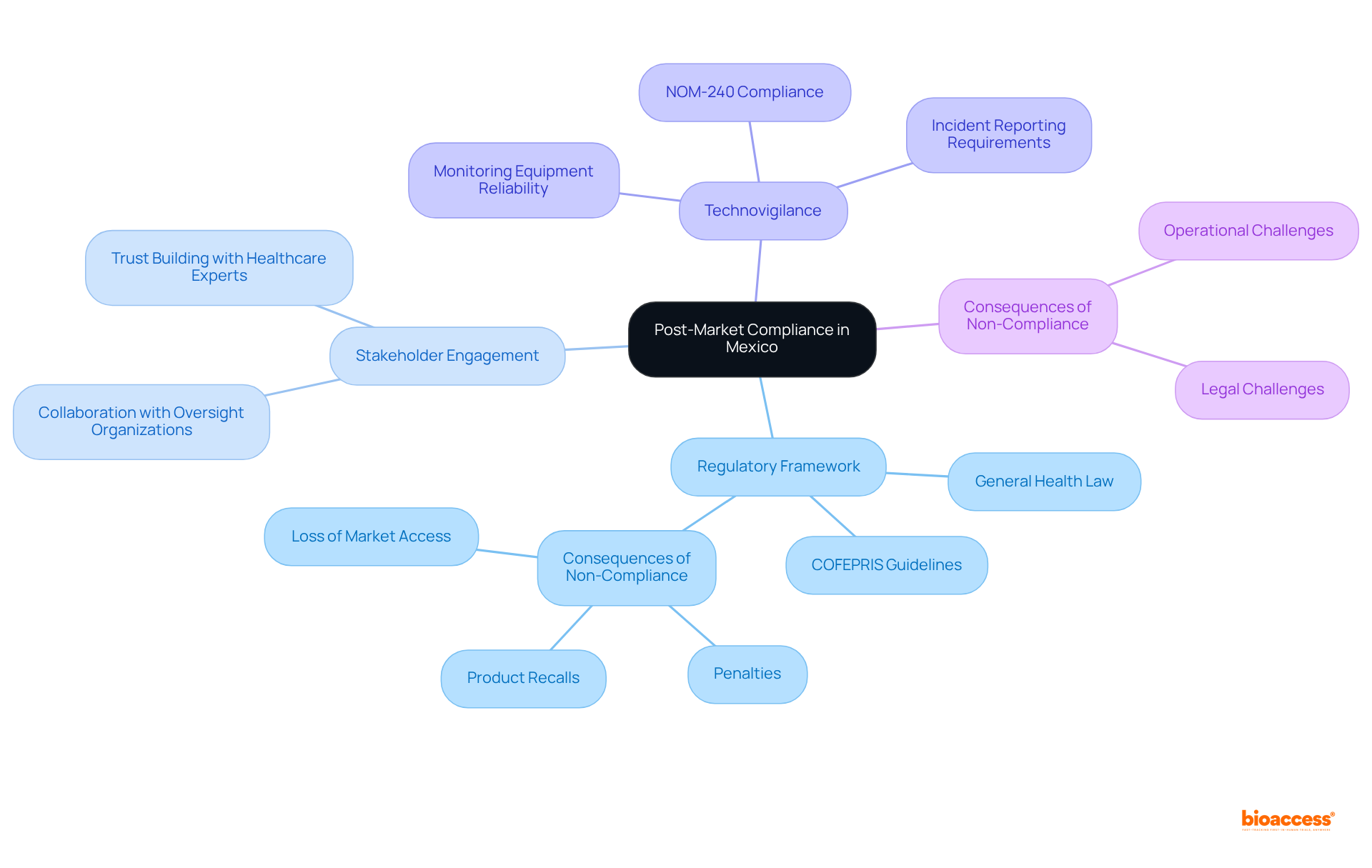
Manufacturers must order post-market compliance package Mexico to effectively navigate the regulatory landscape in Mexico and adhere to several essential requirements.
Sanitary Registration: Securing a sanitary registration from COFEPRIS, Mexico's primary regulatory authority for healthcare instruments, is mandatory for all medical devices. This procedure guarantees that products comply with quality and efficacy standards, with approvals usually obtained in only 4-6 weeks.
Good Manufacturing Practices (GMP): Compliance with GMP standards is crucial for maintaining product quality. Manufacturers must submit comprehensive documentation, including a valid ISO 13485:2016 Audit Certificate and an MDSAP Audit Certificate demonstrating compliance with ISO 13485, to demonstrate adherence to these practices.
Technovigilance: Creating a strong technovigilance system is essential for overseeing equipment performance and promptly reporting any negative incidents, thus ensuring continuous patient protection. As highlighted, 'Prompt adverse event reporting is a commitment to patient well-being and equipment integrity.'
Labeling Requirements: All product labels must comply with COFEPRIS standards, including specific language and content requirements. This includes providing instructions in Spanish in the registration dossier to facilitate user understanding.
Post-Market Surveillance: Developing a comprehensive plan for post-market surveillance is essential. This plan should detail continuous monitoring of device safety and effectiveness, incorporating the order post-market compliance package Mexico, with regular reporting to COFEPRIS to ensure adherence and address any emerging issues.
By proactively engaging with these regulatory requirements, manufacturers can enhance their chances of successful market entry and uphold adherence in Mexico's dynamic healthcare environment.
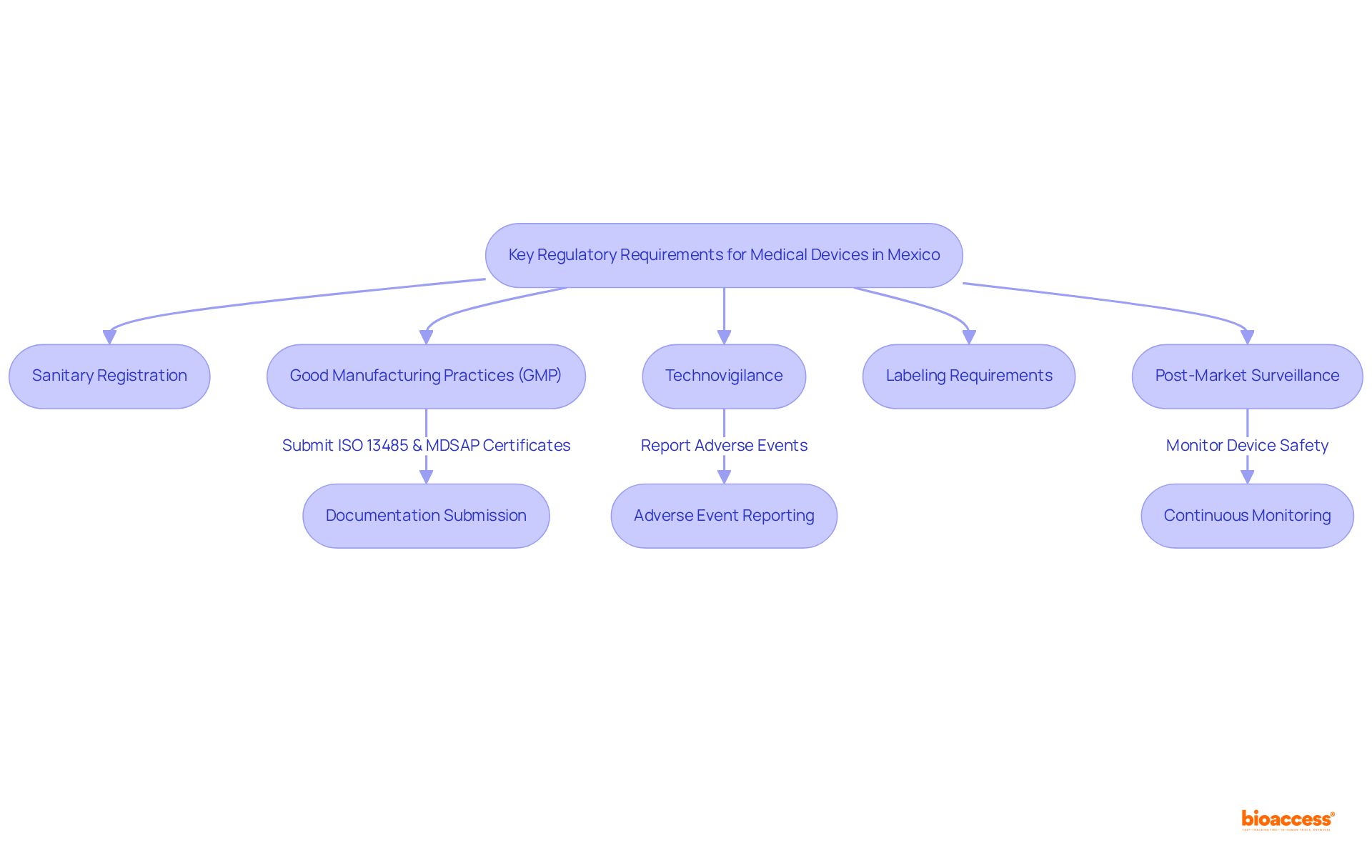
Creating an order post-market compliance package is essential for ensuring continuous conformity in medical device regulation. This process involves gathering all required documentation and establishing systems to order the post-market compliance package Mexico that guarantees compliance. Key steps include:
Documentation: Collect all relevant documents, such as clinical trial data, safety reports, and quality management system (QMS) records. Thorough documentation is vital for demonstrating adherence and ensuring the ongoing safety and functionality of medical equipment. Notably, the FDA receives over 2 million medical device reports annually, highlighting the critical importance of monitoring device performance and safety.
Adherence Strategy: Formulate a comprehensive adherence strategy that outlines how you will meet COFEPRIS requirements, which includes the order post-market compliance package Mexico, detailing timelines and responsibilities. This plan should draw on insights from successful case studies, particularly emphasizing the importance of ordering post-market compliance package Mexico documentation in fulfilling regulatory obligations.
Training: It is imperative that your team is well-versed in regulatory protocols and understands the significance of post-market surveillance. A knowledgeable team is essential for maintaining high standards of safety and efficacy.
Audit Procedures: Implement internal audit procedures to regularly assess compliance with legal requirements. Regular audits are crucial for identifying potential issues early and ensuring that corrective actions are executed promptly.
Reporting Mechanisms: Establish systems to ensure the order post-market compliance package Mexico for the timely reporting of adverse events and other compliance-related information to COFEPRIS. This proactive approach is vital for maintaining equipment reliability and efficiency throughout its lifecycle.
By focusing on these critical components, organizations can adeptly navigate the complexities of post-market adherence, ensuring that their medical products meet the necessary safety and legal standards. As Bioaccess emphasizes, "Effective post-market surveillance documentation is essential for regulatory adherence and ensuring the safety and performance of medical instruments.
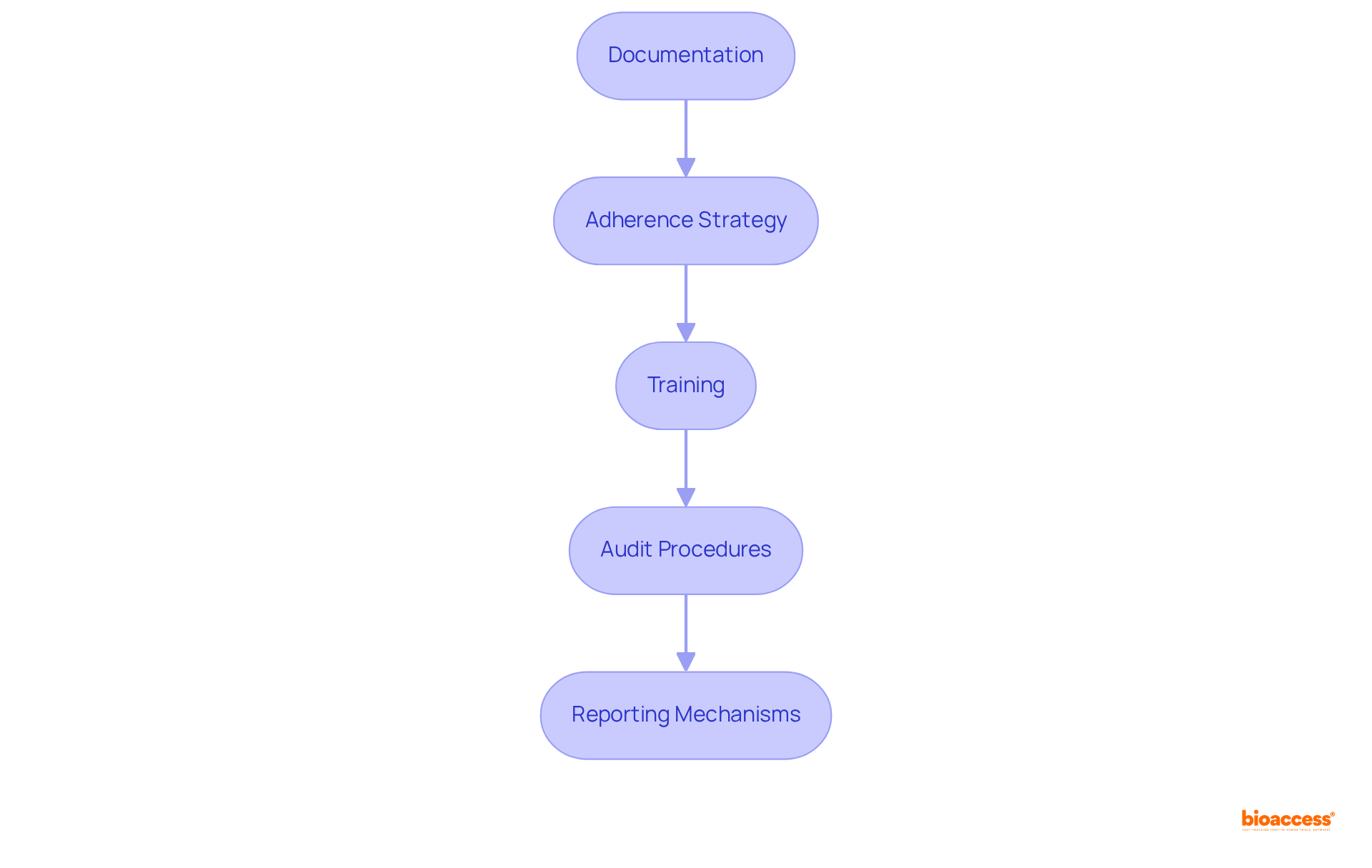
To ensure ongoing compliance and product safety, establishing robust continuous monitoring and reporting mechanisms is essential.
Monitoring Systems: Implement comprehensive systems to continuously track device performance and safety data, ensuring real-time insights into product efficacy. For instance, the global remote patient monitoring market is projected to grow at a compound annual growth rate of about 20% from 2023 to 2028, highlighting the increasing importance of effective monitoring systems.
Data Analysis: Regularly examine the collected information to identify trends or possible concerns. A notable rise in negative event reports can suggest underlying issues that require prompt attention. According to a case study from the University of Pittsburgh Medical Center, implementing remote patient monitoring led to a 76% reduction in readmission rates, showcasing the impact of effective data analysis on patient outcomes.
Reporting Protocols: Develop clear protocols for reporting adverse events to COFEPRIS, including specific timelines and designated responsible parties to ensure accountability and prompt action. As Drew Schiller, CEO of Validic, states, "Every person deserves access to affordable, quality healthcare enabled by advanced digital health solutions, regardless of their location or economic status."
Stakeholder Communication: Maintain open lines of communication with healthcare providers and regulatory bodies. This enables prompt reporting and feedback, which is essential for tackling concerns related to safety efficiently. Wes Haydon, President of CoachCare, emphasizes the importance of collaboration in enhancing patient care through technology.
Review and Update: Regularly assess and revise your adherence strategies based on new regulations, market feedback, and internal audits. This proactive approach helps in adapting to evolving safety standards and enhances overall product reliability. The projected growth of the patient monitoring devices market, expected to reach USD 71.1 billion by 2029, underscores the need for continuous improvement in compliance strategies.
Ensuring post-market compliance in Mexico is not merely a regulatory obligation; it is a crucial aspect of maintaining market authorization and safeguarding patient safety. This article outlines the imperative steps to successfully navigate the complexities of compliance, emphasizing the need for a comprehensive post-market compliance package that adheres to COFEPRIS regulations.
Key insights include:
By securing sanitary registration, adhering to Good Manufacturing Practices, and implementing effective technovigilance, manufacturers can enhance their compliance efforts. Additionally, the necessity of thorough documentation and proactive reporting mechanisms cannot be overstated, as they are vital for demonstrating adherence and ensuring ongoing product safety.
In conclusion, the significance of post-market compliance in Mexico extends beyond mere adherence to regulations; it is essential for fostering trust and reliability in medical devices. Organizations are encouraged to prioritize these compliance strategies, as doing so not only mitigates risks but also enhances their reputation in a rapidly evolving healthcare landscape. By committing to continuous improvement and effective stakeholder collaboration, manufacturers can ensure their products not only meet regulatory standards but also contribute positively to patient outcomes and overall healthcare quality.
What governs post-market compliance in Mexico?
Post-market compliance in Mexico is governed by the regulations established by COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios), which requires continuous monitoring of the reliability and performance of medical devices post-approval.
Why is it important to order a post-market compliance package in Mexico?
Ordering a post-market compliance package in Mexico is essential for tracking adverse events, conducting regular audits, ensuring compliance with reporting requirements, maintaining market authorization, and safeguarding patient safety.
What key components are essential for an effective post-market compliance strategy?
Key components include understanding the regulatory framework (General Health Law and COFEPRIS guidelines), stakeholder engagement, and the implementation of a technovigilance system for monitoring equipment reliability and performance.
What are the consequences of non-compliance with post-market regulations in Mexico?
Non-compliance can lead to severe repercussions such as penalties, product recalls, or loss of market access.
How does stakeholder engagement contribute to post-market compliance?
Collaborating with oversight organizations and healthcare experts enhances adherence to legal standards and improves the trustworthiness of medical products.
What is the significance of the technovigilance system mandated by NOM-240?
The technovigilance system is critical for monitoring equipment reliability and performance, and it is necessary for the prompt reporting of incidents, which is essential for maintaining compliance.
How does the regulatory environment in Mexico affect clinical studies?
Statistics indicate that enrollment for clinical studies in Mexico is 50% faster than in traditional markets, showcasing the efficiency of the regulatory environment.
Why is documentation important in the post-market compliance process?
Documentation, such as the technovigilance report, is necessary for the initial renewal of COFEPRIS registration and is crucial for meticulous regulatory efforts.
How can local compliance experts assist in the approval process?
Collaborating with local compliance experts can streamline the approval process and provide insights into the latest COFEPRIS requirements, enhancing the security and effectiveness of medical products in the market.