


Radiopharmaceutical therapy represents one of the most promising frontiers in precision oncology, yet the path from bench to bedside remains fraught with complex challenges that have created significant knowledge gaps in the field. As the global radiopharmaceuticals market races toward a projected $35.04 billion by 2034 with an 11.45% compound annual growth rate, the clinical trial landscape faces unprecedented pressures to deliver innovative theranostic solutions while navigating regulatory complexities, supply chain constraints, and evolving patient care paradigms. This analysis identifies a critical underexplored area: the integration of real-world evidence (RWE) collection and artificial intelligence-driven methodologies to overcome persistent barriers in radiopharmaceutical clinical development.
Recent analyses reveal that while radiopharmaceutical therapies have demonstrated remarkable efficacy in controlled clinical trials, significant gaps exist in understanding their real-world performance, long-term safety profiles, and optimal integration strategies. The disconnect between clinical trial data and real-world outcomes has become particularly evident as only 0.4% of theranostic studies are conducted in low-income countries and merely 18% in low- and middle-income countries combined, creating a profound geographic disparity in evidence generation.
Moreover, the traditional clinical trial framework struggles to capture the full complexity of radiopharmaceutical administration in diverse healthcare settings. Published extravasation rates in nuclear medicine range from 2% to 38% across different facilities, with some centers reporting rates as high as 44%. This variability, rarely captured in formal clinical trials, underscores the critical need for comprehensive real-world data collection to inform best practices and quality improvement initiatives.
The radiopharmaceutical field has traditionally relied on passive surveillance systems and limited post-market data collection. Unlike conventional pharmaceuticals, where robust pharmacovigilance systems exist, radiopharmaceutical post-market surveillance faces unique challenges. The FDA's MedWatch system and European Databank on Medical Devices remain underutilized for radiopharmaceutical adverse event reporting, with only 21 extravasations documented in the FDA's adverse event system between 1968 and 2019.
Recent innovations in RWE methodology offer promising solutions. A groundbreaking partnership between Cook Medical and the Regenstrief Institute demonstrated how electronic health records (EHR) can be systematically mined to generate meaningful safety and performance data for medical devices. This approach, achieving a 96.7% technical success rate in evaluating embolization coils, provides a template for radiopharmaceutical RWE collection that could transform post-market surveillance practices.
1. Automated EHR Data Extraction
Advanced natural language processing and machine learning algorithms now enable extraction of both structured and unstructured data from clinical records. For radiopharmaceuticals, this could capture:
2. Decentralized Clinical Trial Integration
Hybrid decentralized clinical trials (DCTs) represent a paradigm shift in evidence generation. By enabling patients to participate from home or local facilities, DCTs can:
3. AI-Powered Safety Signal Detection
Artificial intelligence applications in radiopharmaceutical development have shown remarkable potential:
Modern radiopharmaceutical therapy facility showing integration of imaging and treatment capabilities
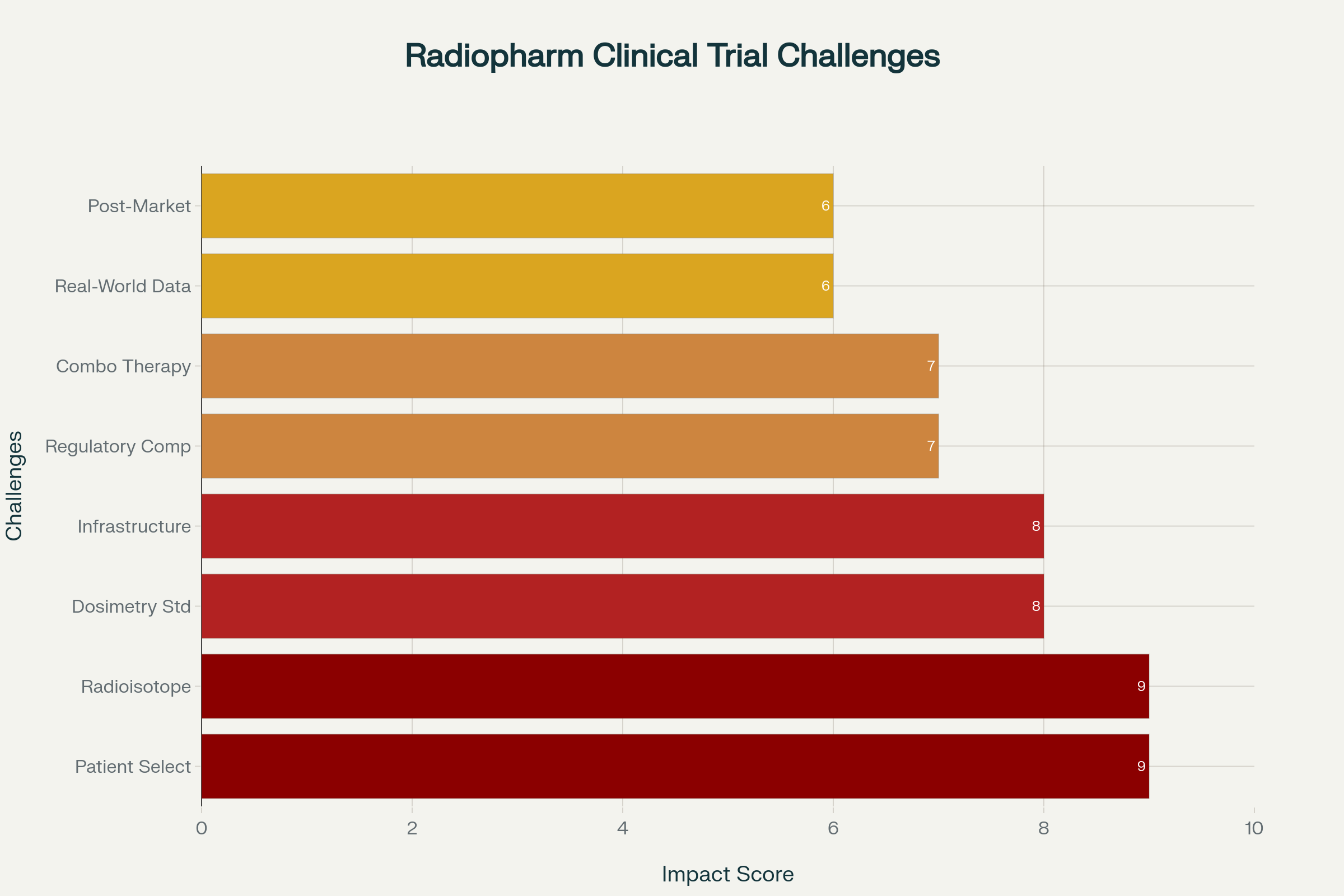
The radiopharmaceutical field faces severe recruitment challenges, with 85% of clinical trials failing to enroll sufficient patients and 80% experiencing delays. RWE offers innovative solutions:
Precision Patient Identification
Decentralized Recruitment Strategies
Current fixed-dose approaches fail to account for individual patient variations, leading to suboptimal outcomes. RWE enables:
Personalized Dosimetry Protocols
Standardization Through Data Aggregation
The short half-lives of therapeutic radioisotopes create unique logistical challenges. Actinium-225, with annual production of only 75 GBq supporting a few hundred patients, exemplifies supply constraints. RWE addresses these challenges through:
Demand Forecasting
Alternative Production Pathway Validation
Complex regulatory requirements, particularly for RAM licensing which can take 1-2 years depending on the country, significantly impact trial timelines. RWE streamlines compliance through:
Proactive Safety Monitoring
Harmonized Reporting Systems
The integration of radiopharmaceuticals with other treatment modalities requires sophisticated trial designs and safety monitoring. RWE facilitates:
Sequential Therapy Optimization
Biological Effective Dose Modeling
Phase 1: Infrastructure Development (Months 1-6)
Phase 2: Pilot Implementation (Months 7-18)
Phase 3: Scale-Up and Integration (Months 19-36)
Phase 4: Continuous Improvement (Ongoing)
The integration of real-world evidence collection with artificial intelligence represents a transformative opportunity for radiopharmaceutical and theranostic clinical development. By addressing critical challenges in patient selection, dosimetry standardization, supply chain management, and regulatory compliance, this approach can accelerate the delivery of life-saving therapies to patients while generating robust evidence for continuous improvement.
Success requires unprecedented collaboration among stakeholders, investment in digital infrastructure, and commitment to data sharing. However, the potential rewards—improved patient outcomes, reduced development costs, and accelerated innovation—justify the effort. As the field stands at this critical juncture, embracing RWE methodologies is not merely an option but an imperative for realizing the full potential of precision radiopharmaceutical therapy.
Q1: How does real-world evidence collection differ from traditional clinical trial data in radiopharmaceutical development?
Real-world evidence captures treatment outcomes in diverse clinical settings beyond controlled trial environments, including variations in administration techniques, patient populations, and healthcare infrastructure. Unlike clinical trials with strict protocols, RWE reflects actual practice patterns, revealing extravasation rates ranging from 2-38% across facilities and identifying quality improvement opportunities that controlled studies miss.
Q2: What are the main technological requirements for implementing RWE collection in radiopharmaceutical programs?
Key requirements include: electronic health record systems with natural language processing capabilities for unstructured data extraction; secure data warehouses with HIPAA-compliant architecture; machine learning platforms for pattern recognition and predictive analytics; interoperability standards enabling multi-site data aggregation; and radiation-specific tracking systems for dosimetry and safety monitoring.
Q3: How can smaller radiopharmaceutical programs participate in RWE initiatives without extensive resources?
Smaller programs can leverage cloud-based platforms and consortiums to share infrastructure costs. Participating in existing registries like those managed by professional societies reduces individual burden. Open-source tools and standardized protocols from initiatives like the IAEA guidance documents provide accessible frameworks. Partnering with academic institutions or CROs specializing in RWE can provide expertise without building internal capabilities.
Q4: What regulatory considerations apply to using RWE for radiopharmaceutical development?
Regulatory agencies increasingly accept RWE for post-market surveillance and label expansions. Key considerations include: ensuring data quality meets FDA and EMA standards for regulatory decision-making; implementing audit trails and data integrity measures; obtaining appropriate patient consent for data use; complying with radiation-specific reporting requirements; and coordinating with both drug and nuclear regulatory authorities.
Q5: How does AI-powered RWE collection address the unique challenges of alpha-emitting radiopharmaceuticals?
AI algorithms can predict complex decay chain behaviors and daughter radionuclide redistribution patterns that traditional methods struggle to model. Machine learning identifies optimal patient populations for alpha therapies by analyzing genomic and clinical data. Automated safety monitoring detects rare adverse events across small patient populations. Predictive models optimize limited isotope supplies by forecasting treatment outcomes.
Bogsrud TV, Lowe VJ. Normal variants and pitfalls in whole-body PET imaging with 18F FDG. Applied Radiology. 2006;35(6):16-30. DOI: 10.37549/AR1432
Sartor O, et al. Trial data demonstrated clinical benefit in delaying disease progression in eligible patients. Mayo Clinic Proceedings. 2024. Cited in Novartis press release March 28, 2025.
McIntosh C, Abele J. Frequency of interstitial radiotracer injection for patients undergoing bone scan. 79th Annual Scientific Meeting of the Canadian Association of Radiologists; Montreal, Quebec. 2016.
Wang SJ. Recent advances in clinical trial design considerations in Theranostics. Contemporary Clinical Trials. 2020;96:106100. DOI: 10.1016/j.cct.2020.106100
Society of Nuclear Medicine & Molecular Imaging Annual Meeting. Radiopharmaceuticals reshaping oncology trials through integrated imaging. July 1, 2025. Available from: medpace.com
Crowley JR, et al. Radiopharmaceutical administration practices—Are they best practice? Frontiers in Nuclear Medicine. 2023;3:1244660. DOI: 10.3389/fnume.2023.1244660
Tagawa S, et al. Phase II CONVERGE-01 trial of Ac-225 rosopatamab tetraxetan. Journal of Clinical Oncology. 2024;42(13):1455-1464.
ASCO Abstract 11575. Global inequities in sarcoma clinical trials. Journal of Clinical Oncology. 2025;43(16_suppl):11575.
Parexel. Advancing radiopharmaceutical development: Integrated clinical trial expertise. May 28, 2025. Available from: parexel.com
Production and Supply of α-Particle–Emitting Radionuclides. Journal of Nuclear Medicine. 2021;62(7):935-942.
Frontiers in Medicine. Overview of phase 3 radiopharmaceutical therapy clinical trials. February 18, 2025;12:1549676.
Alpha-emitting radionuclides: Current status and future perspectives. Pharmaceuticals. 2024;17(1):76. DOI: 10.3390/ph17010076
Abdera Therapeutics AACR presentation on DLL3-targeting radiopharmaceutical ABD-147. April 23, 2025. Press release.
Production and regulatory issues for theranostics. Journal of Nuclear Medicine. August 2021;62(8):1040-1046.
Radiopharmaceutical administration practices survey. Frontiers in Nuclear Medicine. 2023;3:1244660.
Design and conduct of theranostic trials in neuro-oncology. Neuro-Oncology. 2024;26(Supplement_9):S199.
MUHC first patients in Canada enrolled in radioligand therapy trial. May 27, 2025. McGill University Health Centre news release.
Mayo Clinic treats first US patient with novel radiopharmaceutical for breast cancer. August 1, 2025. Mayo Clinic Newsnetwork.
Global challenges in provision of theranostic services. Nuklearmedizin. 2025;64(2):89-98. DOI: 10.1055/s-0045-1807251
Nature. Radiopharmaceuticals and applications in medicine. January 3, 2025. DOI: 10.1038/s41392-024-02041-6
BAMF Health. Celebrating World Theranostics Day 2025. March 27, 2025. Available from: bamfhealth.com
VHIO. Radioligand therapy demonstrates improved clinical benefit in GEP-NETs. March 6, 2025. Press release.
McGuire Woods. Radiopharmaceutical Industry Update Q4/Q1 2024-2025. May 6, 2025.
Hobbs RF, et al. Dosimetry in Radiopharmaceutical Therapy. Journal of Nuclear Medicine. 2022;63(1):83-91.
Silva DJ, et al. Decentralized clinical trials in early drug development. Frontiers in Medicine. 2024;11:1355912.
AI transforming radiopharmaceutical research. MEDI Thailand. April 8, 2025. Available from: medi-thailand.com
Combination therapy enhancing radiopharmaceutical efficacy. Pharmaceuticals. 2021;14(5):423.
NCBI. Decentralized clinical trials and digital health technologies. February 9, 2024. Available from: ncbi.nlm.nih.gov
Artificial intelligence and future of radiopharmaceuticals. European Journal of Nuclear Medicine. 2021;48(9):2809-2815.
Miederer M, et al. Alpha-emitting radionuclides: Current status. Pharmaceuticals. 2024;17(1):76.
Postmarket surveillance of neuroendovascular devices. Stroke: Vascular and Interventional Neurology. 2024;4(3):e001081.
Parexel. Clinical trial feasibility and site selection for radiopharmaceuticals. 2025.
Radiobiological optimization of combination therapy. International Journal of Radiation Oncology. 2014;89(3):628-636.
Real-world data supporting medical device post-market surveillance. BMC Medical Informatics and Decision Making. 2024;24:263.
OpenClinica. Simplifying patient recruitment for clinical trials. September 26, 2024.