


The essential steps for success in preparing for a pre-IND meeting are crucial to understanding the meeting's purpose and ensuring effective engagement with the FDA.
Thorough preparation, including clarity in documentation and targeted inquiries, is paramount for facilitating a smoother drug development process. Are you ready to take the necessary steps to ensure your success in this critical phase?
Navigating the complexities of drug development necessitates a strategic approach, particularly regarding pre-IND meetings with the FDA. These consultations are pivotal, providing sponsors with an invaluable opportunity to clarify regulatory expectations and refine their development plans. By grasping the essential steps for a successful pre-IND meeting, stakeholders can significantly bolster their chances of a seamless IND submission process. Yet, what are the key elements that can make or break this critical interaction with regulatory authorities?
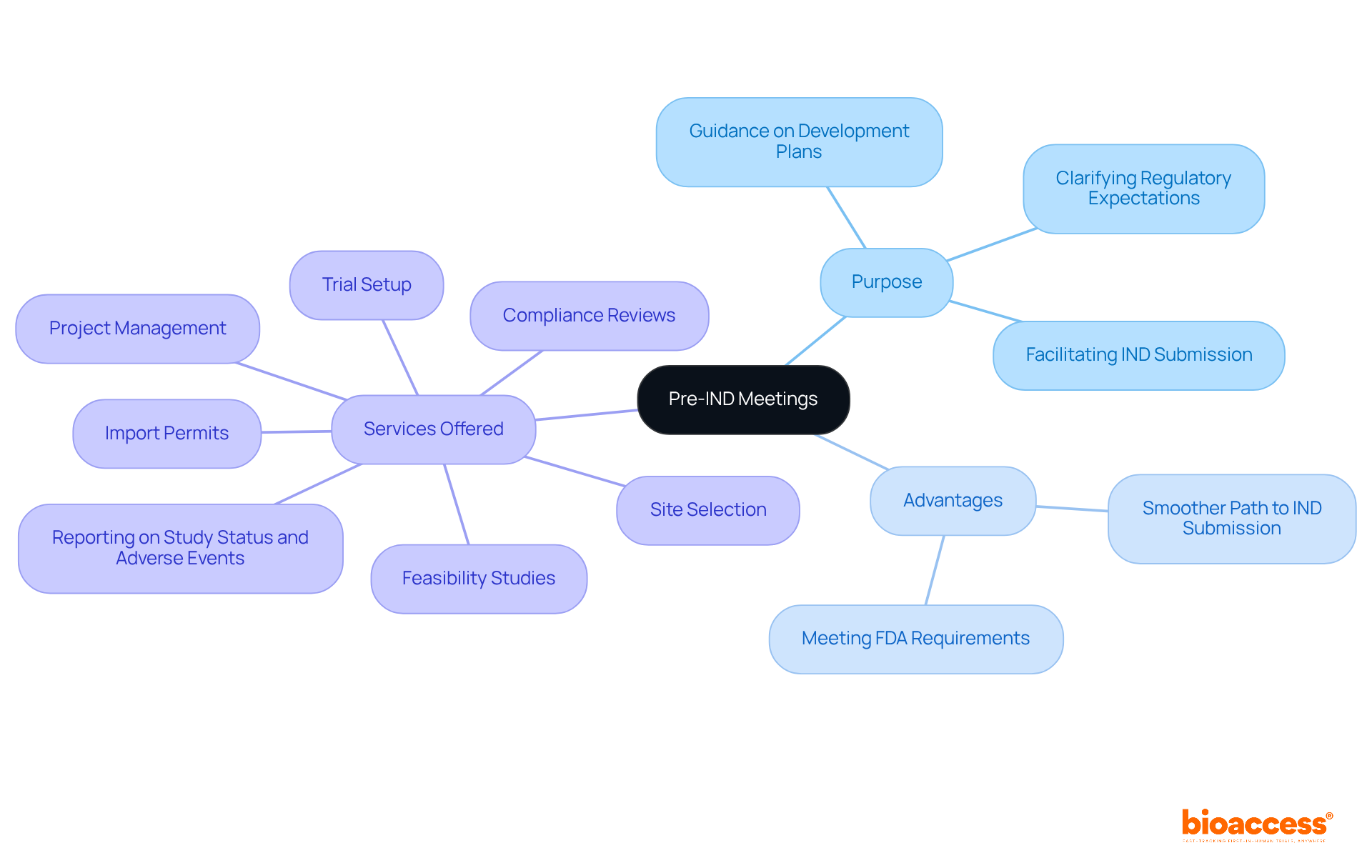
Pre-IND consultations represent formal discussions with the FDA that occur early in the drug development process. Their primary purpose is to provide sponsors with essential guidance on their development plans, which includes the design of preclinical studies and initial clinical trials. Understanding this objective is crucial for formulating the right questions and preparing adequately for the meeting.
These consultations are particularly advantageous for clarifying regulatory expectations and ensuring that the proposed studies meet FDA requirements, ultimately facilitating a smoother path to IND submission.
At bioaccess®, we excel in comprehensive trial management services, encompassing:
Our expertise in regulatory navigation, particularly within Latin America, positions us as a leading CRO for medical device research trials, ensuring that Medtech startups receive tailored solutions to address their unique needs during the Pre-IND process.
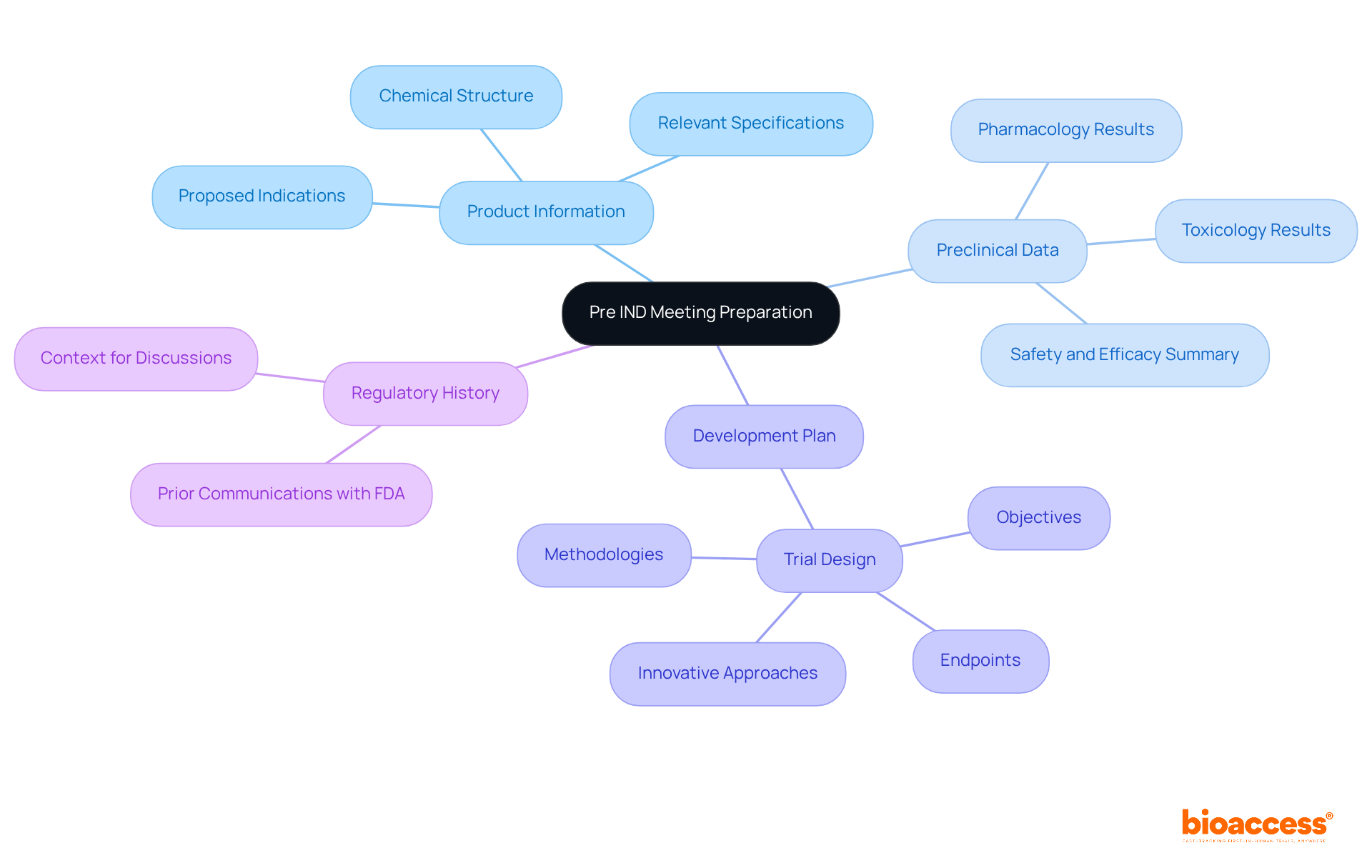
To effectively prepare for a pre IND meeting, it is crucial to meticulously compile all necessary documentation. This includes:
Ensuring that all documents are current, well-organized, and easily accessible will significantly enhance the productivity of the pre IND meeting. Common documentation issues faced by sponsors often stem from incomplete or unclear submissions, which can lead to misunderstandings or delays. Therefore, a thorough review of the documentation checklist prior to the pre IND meeting is recommended to avoid these pitfalls.
Furthermore, examples of thorough development plans can act as useful references, demonstrating the level of detail and clarity anticipated by regulatory authorities. Engaging with regulatory experts, such as those from Bioaccess who specialize in clinical trial management and regulatory affairs, can provide insights into essential documentation requirements, further strengthening the preparation process. Bioaccess offers services such as feasibility studies and site selection, which can help address the challenges faced by medical device startups in navigating regulatory hurdles. Additionally, building a relationship with FDA review division personnel through open dialogue during the session can lead to a more fruitful discussion. It is also recommended to designate a timekeeper to oversee time during the gathering and keep discussions centered on key objectives. Ultimately, organizing inquiries to elicit clear 'yes' or 'no' responses can improve the effectiveness of communication with the FDA.
The briefing package must be comprehensive and well-organized, typically including:
Incorporating bioaccess®'s accelerated regulatory approval process, which allows for approvals in just 6-8 weeks compared to the typical 6-12 months in the US/EU, significantly enhances the briefing package. This efficiency is particularly beneficial for treatment-naive cardiology or neurology cohorts, enabling faster patient enrollment—up to 50% quicker than Western sites.
Additionally, ensure that the briefing package reflects bioaccess®'s comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. This holistic approach not only streamlines the process but also effectively prepares the FDA-ready data submission. Providing a detailed overview of these services aligns the package with the FDA's expectations for thoroughness and clarity.
Submit the briefing package at least 30 days before the gathering to allow the FDA sufficient time to review the materials.

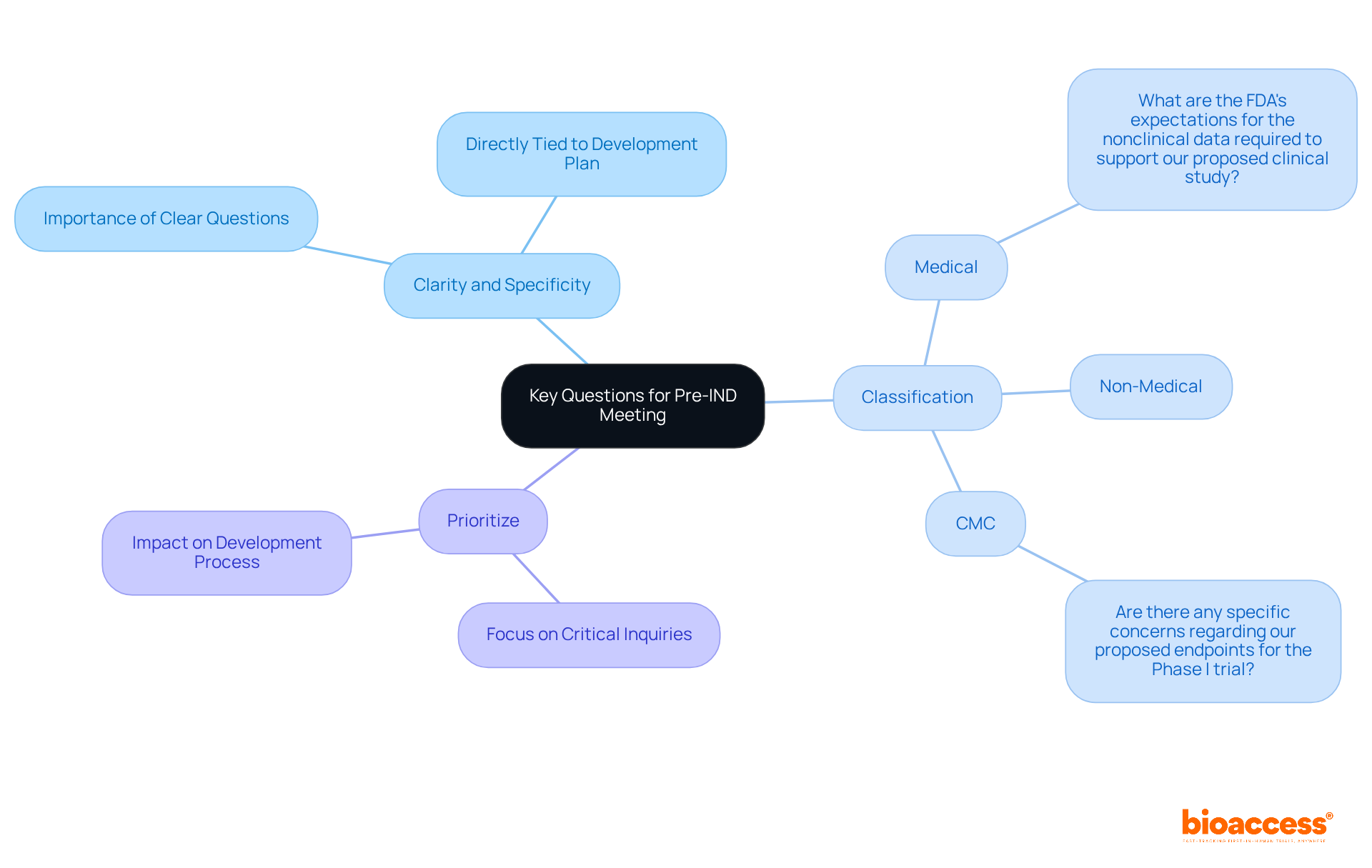
When preparing questions for the pre-IND meeting, it is crucial to consider the following elements:
Examples of pertinent questions include:
This approach will foster a focused and productive dialogue in the pre-IND meeting with FDA representatives.
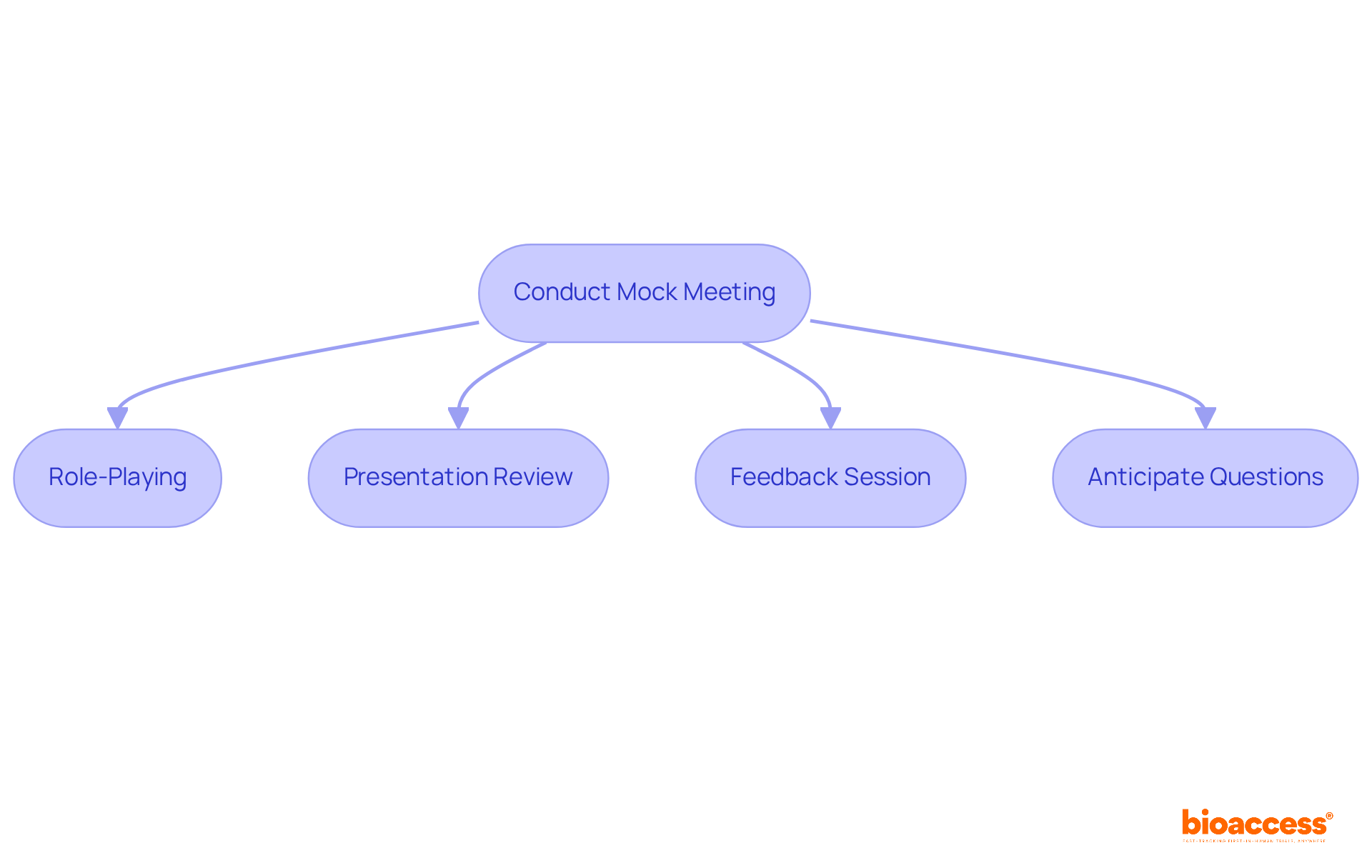
To enhance the effectiveness of the pre IND meeting, it is essential to conduct a mock session with your team. This practice session should include the following components:
This thorough preparation will help ensure that the actual meeting runs smoothly, equipping the team to effectively address any concerns raised by the FDA.
Preparing for a pre-IND meeting is a pivotal step in the drug development process, serving as a crucial opportunity for sponsors to engage with the FDA and clarify their development plans. Understanding the purpose of these meetings and following a structured approach enables companies to effectively navigate the complexities of regulatory expectations and significantly enhance their chances of a successful IND submission.
Key insights from the article emphasize the importance of thorough documentation, including:
Creating a detailed briefing package and formulating specific questions for discussion are essential strategies that can lead to productive dialogues with FDA representatives. Additionally, conducting mock meetings allows teams to refine their presentations and anticipate potential inquiries, ensuring they are well-prepared for the actual meeting.
Ultimately, the significance of pre-IND meetings cannot be overstated, as they lay the groundwork for a successful drug development journey. By investing time and effort into meticulous preparation, companies can foster a collaborative relationship with regulatory authorities, paving the way for innovative solutions that can benefit patients worldwide. Taking these essential steps will not only enhance the quality of the pre-IND meeting but also contribute to the overall success of the drug development process.
What is the purpose of Pre-IND meetings?
Pre-IND meetings are formal discussions with the FDA that occur early in the drug development process. Their primary purpose is to provide sponsors with guidance on development plans, including preclinical studies and initial clinical trials, and to clarify regulatory expectations.
How can Pre-IND meetings benefit sponsors?
These meetings help ensure that proposed studies meet FDA requirements, facilitating a smoother path to IND submission and clarifying regulatory expectations.
What types of services does bioaccess® offer regarding trial management?
Bioaccess® offers comprehensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting on study status and adverse events.
What documentation is required to prepare for a Pre-IND meeting?
Required documentation includes product information, preclinical data, a development plan, and regulatory history. This documentation should be current, well-organized, and easily accessible.
What should be included in the product information for the Pre-IND meeting?
Product information should provide comprehensive details about the drug, including its chemical structure, proposed indications, and relevant specifications.
Why is summarizing preclinical data important for the Pre-IND meeting?
Summarizing preclinical data, including pharmacology and toxicology results, demonstrates the drug's safety and efficacy, which is crucial for discussions with the FDA.
How can sponsors avoid common documentation issues before the Pre-IND meeting?
Sponsors can avoid issues by ensuring that all documents are complete, clear, and organized, and by reviewing a documentation checklist prior to the meeting.
What role do regulatory experts play in preparing for the Pre-IND meeting?
Regulatory experts, such as those from Bioaccess, can provide insights into essential documentation requirements and help strengthen the preparation process.
How can building a relationship with FDA personnel benefit the Pre-IND meeting?
Engaging in open dialogue with FDA review division personnel can lead to more fruitful discussions during the meeting.
What strategies can improve communication effectiveness during the Pre-IND meeting?
Organizing inquiries to elicit clear 'yes' or 'no' responses and designating a timekeeper to oversee discussions can enhance communication effectiveness.