


This article presents a comprehensive step-by-step guide for securing a Clinical Research Associate (CRA) contract, detailing essential responsibilities, necessary qualifications, effective application strategies, and networking techniques.
It is crucial to understand the CRA's role and obtain relevant certifications.
Tailoring applications and actively engaging with industry professionals are vital for enhancing employability and succeeding in the competitive clinical research landscape.
By following these strategies, aspiring CRAs can significantly improve their prospects in this dynamic field.
Navigating the path to becoming a Clinical Research Associate (CRA) presents a journey abundant with opportunities and challenges. With the demand for skilled professionals in the clinical research field on the rise, understanding how to secure a CRA contract is essential for aspiring candidates. This guide outlines not only the necessary qualifications and certifications but also delves into effective strategies for identifying and applying for positions. Furthermore, it highlights the critical role networking plays in career advancement. Given the competitive landscape and evolving industry standards, what are the key steps one must take to stand out and successfully land a CRA contract?
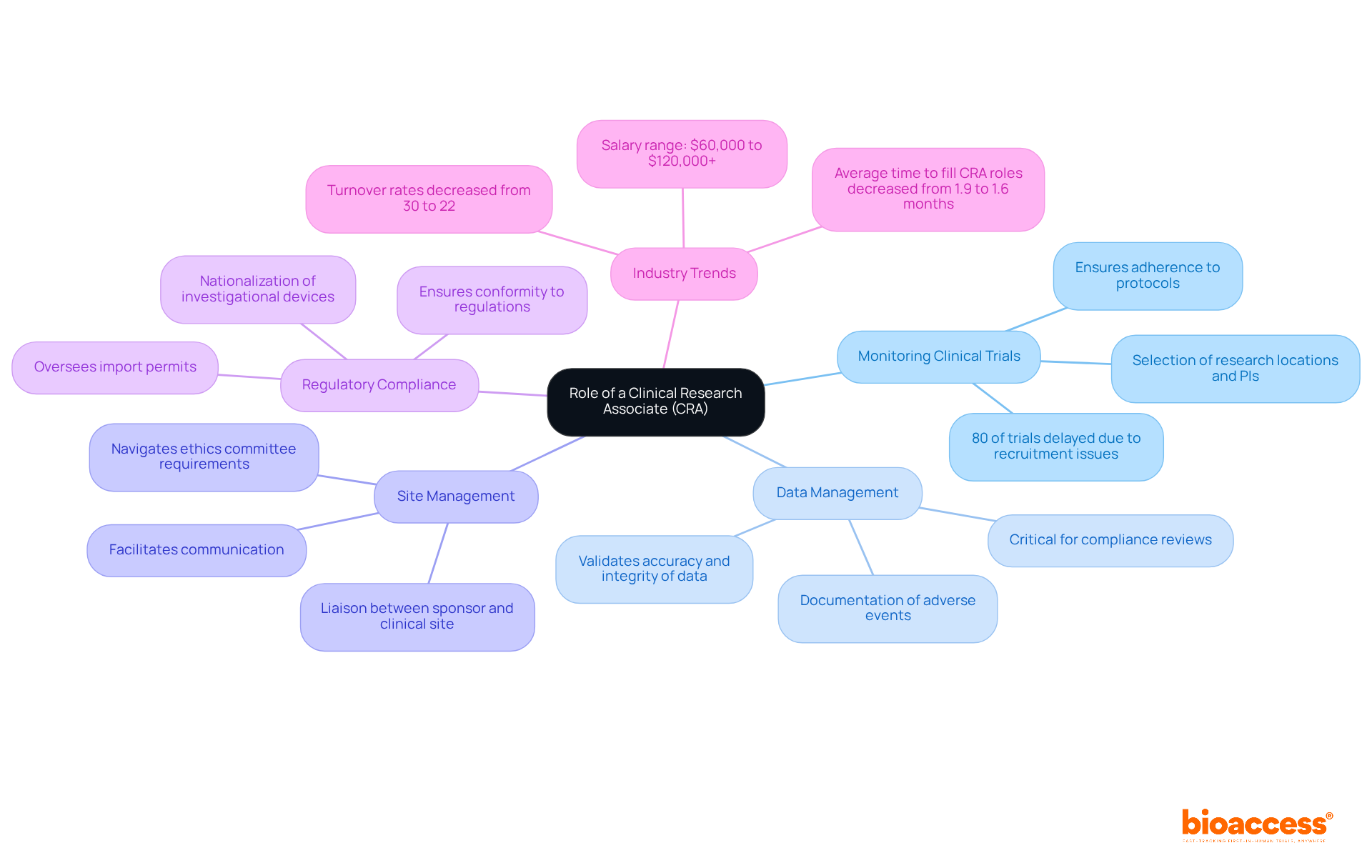
A Clinical Research Associate (CRA) plays a crucial role in the study process, especially in relation to the clinical research associate contract for the comprehensive study management services offered by bioaccess. Their primary responsibilities encompass several key areas:
Monitoring Clinical Trials: CRAs ensure that clinical trials are conducted in accordance with the protocol, Good Clinical Practice (GCP), and regulatory requirements. This oversight is vital, especially considering that 80% of experiments encounter delays due to recruitment challenges, underscoring the necessity for efficient management in this field. Their involvement in the clinical research associate contract is essential in determining the viability and selection of research locations and primary investigators (PIs), ensuring that studies are organized effectively from the outset.
Data Management: They validate the accuracy and integrity of the information collected during experiments, ensuring that all details are meticulously documented and can withstand scrutiny. This is particularly critical in the context of compliance reviews and reporting on study status, inventory, and adverse events.
Site Management: CRAs act as the primary liaison between the sponsor and the clinical site, facilitating communication and promptly addressing any issues that may arise. Their role is vital in the setup and approval process, which includes navigating the requirements of ethics committees and health ministries.
Regulatory Compliance: They guarantee that all aspects of the study conform to local and international regulations, which is essential for the study's success and the safety of participants. This includes overseeing import permits and the nationalization of investigational devices, which are critical components of the management process.
The significance of clinical research associate contracts in research studies is paramount; they are integral to ensuring that studies are executed efficiently and effectively. With turnover rates for CRAs decreasing from 30% in 2022 to 22% in 2024, the industry is experiencing a trend toward improved retention, highlighting the growing recognition of their value. Furthermore, the average time to fill CRA roles has diminished from 1.9 months in 2022 to 1.6 months in 2024, reflecting enhanced recruitment strategies that can alleviate turnover issues. Additionally, numerous trial activities can now be conducted remotely without compromising quality, further advancing the role of CRAs in contemporary research. Understanding these responsibilities will assist you in identifying the skills and experiences to emphasize in your applications and interviews for a clinical research associate contract, positioning you as a strong candidate in this competitive field. CRAs can anticipate earning between $60,000 and $120,000+ per year, making this a lucrative career path.
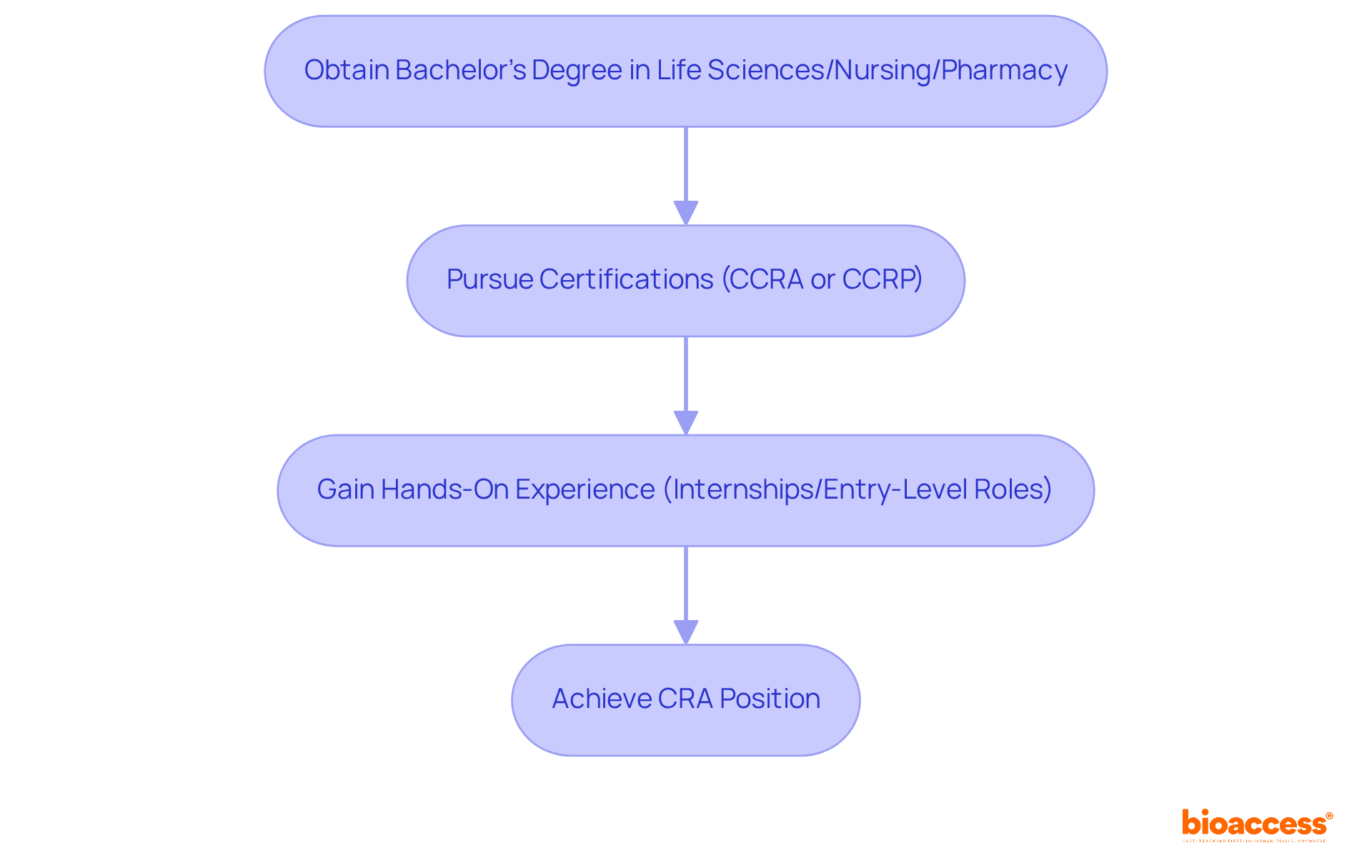
To secure a position as a Clinical Research Associate (CRA), candidates typically need to meet specific qualifications that enhance their employability and readiness for the role. A bachelor's degree in life sciences, nursing, pharmacy, or a related field is often essential. Although some employers might favor applicants with a master's degree, a strong background in health-related fields is crucial for comprehending research processes.
Achieving certifications such as the Certified Clinical Research Associate (CCRA) from the Association of Clinical Research Professionals (ACRP) or the Clinical Research Associate Certification (CCRP) from the Society of Clinical Research Associates (SoCRA) can greatly influence career success. While certification is not mandatory, it can enhance job prospects and lead to higher salaries. Statistics indicate that certified CRAs often enjoy better job prospects and higher salaries, with median earnings reported at $100,890 per year, compared to an average salary of $80,732 reported by Glassdoor. Furthermore, approximately 40% of CRAs hold certifications, underscoring their importance in the competitive job market.
Acquiring hands-on experience via internships or entry-level roles in research is invaluable. Positions like Study Coordinator (CRC) or Trial Assistant (CTA) offer vital skills and understanding of the trial process. Many successful CRAs begin their careers in these positions, which help them develop a comprehensive understanding of regulatory requirements and study management. For instance, to obtain ACRP certification, candidates must complete 3,000 hours of qualifying work experience, highlighting the importance of gaining relevant experience.
By focusing on these qualifications and pursuing relevant certifications, aspiring CRAs can enhance their employability and demonstrate their commitment to excellence in the field. As exemplified by Christina’s journey from a CRA to an Operations Manager, the right qualifications can result in substantial career progression.
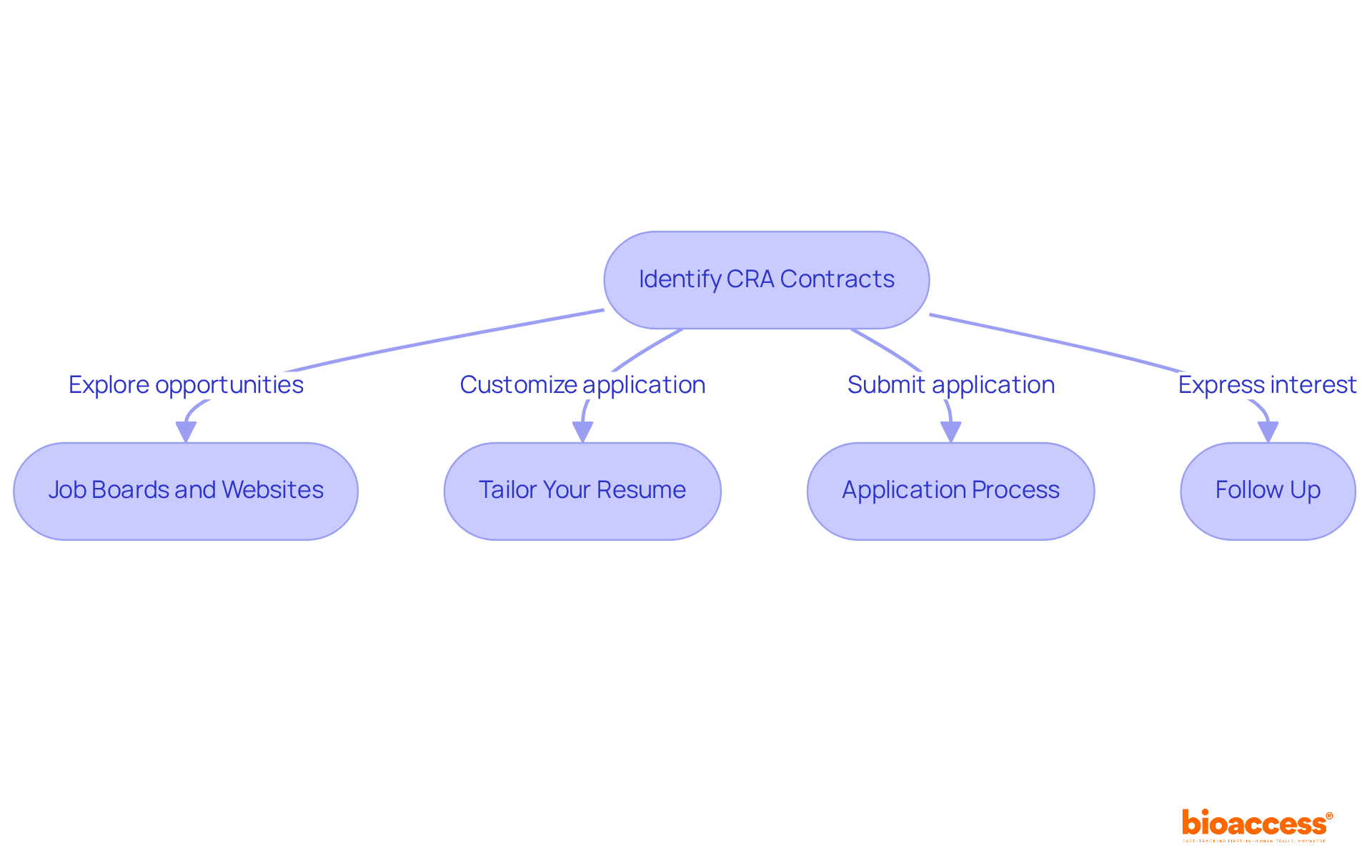
To effectively identify and apply for Clinical Research Associate (CRA) contracts, consider the following strategies:
By actively engaging in these steps, you enhance your chances of securing a position in the competitive CRA landscape, where the demand for qualified professionals is expected to rise significantly in the coming years.
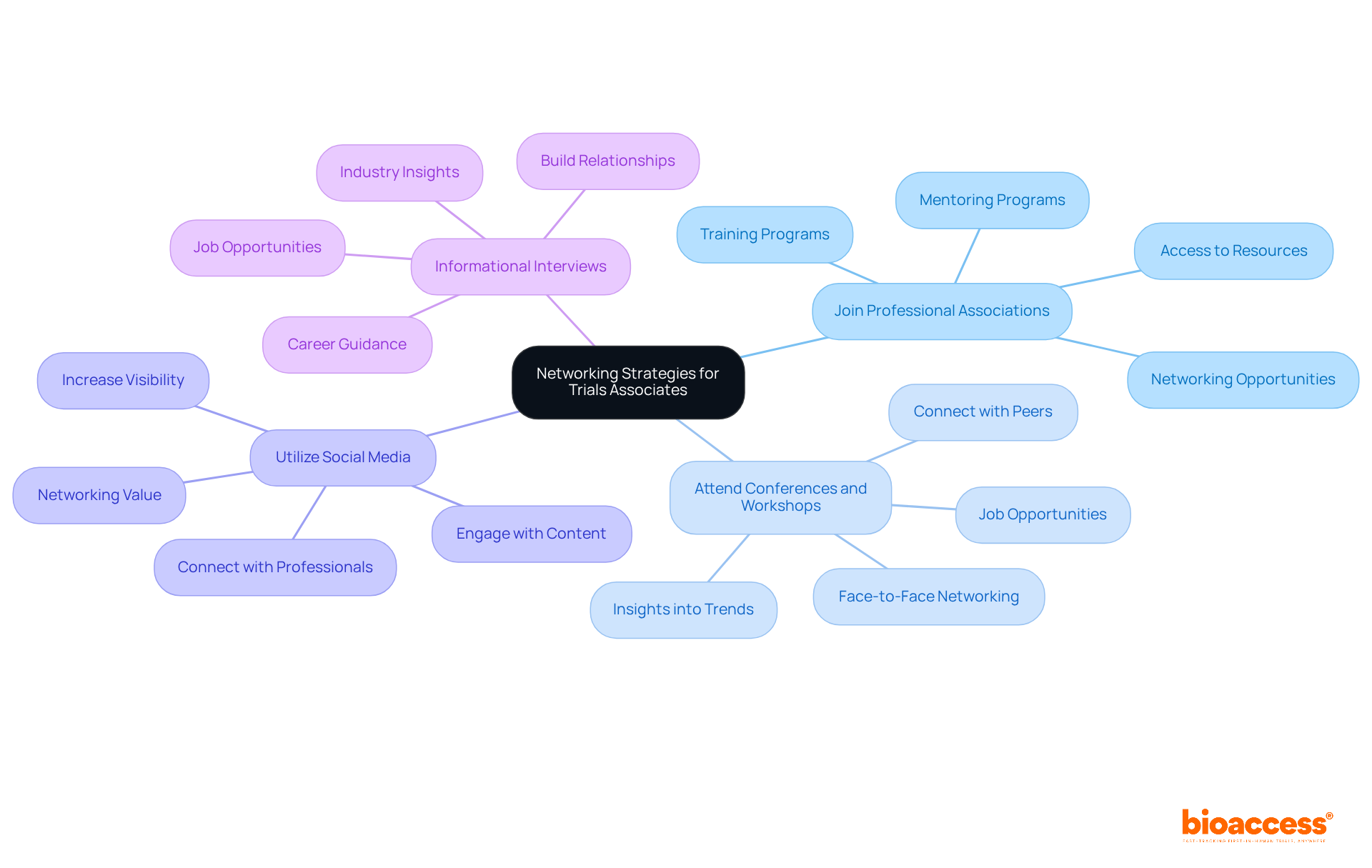
Establishing a robust professional network is crucial for advancing your career as a Trials Associate. Here are effective strategies to enhance your networking efforts:
Join Professional Associations: Becoming a member of organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA) can significantly benefit your career. These associations provide access to valuable resources, training programs, and networking opportunities that can enhance your professional development. Studies indicate that members often experience improved career advancement prospects, with many employers recognizing the value of association membership as equivalent to additional experience. Furthermore, organizations like ACRP and DIA offer formal mentoring programs that can further support your career growth.
Attend Conferences and Workshops: Engaging in industry conferences, workshops, and webinars presents an excellent opportunity to connect with peers and industry leaders. These events not only offer insights into the latest trends but also facilitate valuable connections that can lead to future job opportunities. Networking at such events can be particularly impactful; nearly 100% of professionals believe that face-to-face meetings are essential for maintaining long-term business relationships. The medical research market globally is projected to exceed $80 billion by 2025, underscoring the sector's expansion and the significance of efficient networking.
Utilize Social Media: Leverage platforms like LinkedIn to connect with other professionals in the research field. Actively sharing relevant content and engaging with posts can significantly increase your visibility and help you establish meaningful connections. With over 1 billion users on LinkedIn, the platform serves as a powerful tool for networking and career progression. Notably, 79% of professionals agree that networking is valuable for career advancement, making it essential to utilize these platforms effectively.
Informational Interviews: Reach out to professionals in your desired field for informational interviews. This approach not only provides insights into the industry but also helps you build relationships that may lead to job opportunities. Engaging with experienced professionals can offer guidance and support, reinforcing the importance of a proactive networking strategy.
By actively networking and leveraging these strategies, you can enhance your career prospects and remain informed about opportunities related to the clinical research associate contract.
Securing a Clinical Research Associate (CRA) contract is a multifaceted process that demands a profound understanding of the role, relevant qualifications, and effective networking strategies. A comprehensive exploration of the CRA's responsibilities, the significance of certifications, and the techniques to identify and apply for contracts reveals that a strategic approach is indispensable for success in this competitive field.
The critical nature of the CRA's role encompasses:
Furthermore, obtaining the necessary qualifications and certifications, such as the CCRA or CCRP, significantly enhances a candidate's job prospects and potential earnings. It is also essential to tailor applications, follow up with hiring managers, and leverage professional networks to uncover job opportunities.
As the demand for skilled CRAs continues to escalate, aspiring professionals must proactively engage in building their qualifications and networks. By taking these steps, candidates can position themselves effectively within the clinical research landscape, ultimately contributing to advancements in medical research and patient care. Embracing this proactive approach not only leads to career growth but also fosters a thriving environment for innovation in clinical trials.
What is the primary role of a Clinical Research Associate (CRA)?
A CRA plays a crucial role in monitoring clinical trials, ensuring compliance with protocols, Good Clinical Practice (GCP), and regulatory requirements, as well as managing data and facilitating communication between sponsors and clinical sites.
What are the main responsibilities of a CRA?
The main responsibilities of a CRA include monitoring clinical trials, managing data integrity, acting as a liaison between sponsors and clinical sites, and ensuring regulatory compliance throughout the study process.
How do CRAs ensure the accuracy of data collected during clinical trials?
CRAs validate the accuracy and integrity of the information collected during experiments, ensuring meticulous documentation that can withstand scrutiny, which is essential for compliance reviews and reporting.
What role do CRAs play in site management?
CRAs serve as the primary liaison between the sponsor and the clinical site, facilitating communication and promptly addressing any issues, as well as assisting in the setup and approval processes involving ethics committees and health ministries.
Why is regulatory compliance important in clinical research?
Regulatory compliance is crucial as it ensures that all aspects of the study conform to local and international regulations, which is essential for the study's success and the safety of participants.
What trends are observed in the turnover rates and recruitment of CRAs?
Turnover rates for CRAs have decreased from 30% in 2022 to 22% in 2024, and the average time to fill CRA roles has improved from 1.9 months in 2022 to 1.6 months in 2024, indicating enhanced recruitment strategies and improved retention.
Can trial activities be conducted remotely, and how does this affect CRAs?
Yes, numerous trial activities can now be conducted remotely without compromising quality, which further advances the role of CRAs in contemporary research.
What is the earning potential for CRAs?
CRAs can anticipate earning between $60,000 and $120,000+ per year, making it a lucrative career path.