


Securing an FDA certificate for medical devices necessitates a comprehensive understanding of the regulatory pathways, documentation requirements, and compliance processes. This article outlines specific steps essential for success:
Adherence to these guidelines significantly enhances the likelihood of a successful application and ensures market readiness. The importance of understanding these processes cannot be overstated, as they form the backbone of a successful entry into the medical device market.
Navigating the complex landscape of FDA certification for medical devices presents a formidable challenge for manufacturers and clinical research professionals. Understanding the intricate requirements and pathways is not just beneficial; it is essential, as the stakes are high—delays or rejections can severely hinder market entry and limit patient access to innovative solutions.
This guide offers a step-by-step approach to securing an FDA certificate, emphasizing crucial strategies and insights that can streamline the approval process.
How can companies effectively maneuver through these regulatory hurdles to ensure their medical devices not only meet safety standards but also reach the market promptly?
The FDA (Food and Drug Administration) plays a vital role in overseeing the FDA certificate for medical devices, ensuring that medical equipment in the United States meets safety and effectiveness standards before entering the market. Medical instruments are categorized into three groups according to their risk levels:
Each class has unique regulatory requirements that must be met for authorization. For example, while Class I products often qualify for exempt status, Class II products usually necessitate a pre-market notification (PMN) application, and Class III products must undergo the more stringent pre-market authorization (PMA) process.
Adhering to FDA guidelines is essential for securing an FDA certificate for a medical device; failure to comply can lead to considerable delays or outright rejections in the validation process. Recent statistics show that up to 75% of preliminary clinical testing for equipment has moved outside the United States, largely because of the prolonged regulatory timelines linked to FDA approvals. The average time to issue a decision on FDA 510(k) applications is approximately 147 days, but this timeline can extend further due to requests for additional information, complicating planning for clinical research.
To navigate this complex landscape effectively, it is essential to utilize the FDA's resources, including their website and guidance documents, to stay updated on the latest regulations and requirements for obtaining an FDA certificate for a medical device. Timely and consistent communication with the FDA is recommended to prevent obstacles that could squander time and resources, facilitating a more seamless journey to market for groundbreaking medical products.
In this context, partnering with bioaccess®, a leading CRO in Latin America, can significantly enhance the efficiency of the clinical trial process. Bioaccess® specializes in accelerating clinical study results, providing expertise in regulatory navigation, clinical research site activation, patient recruitment, and trial data delivery. Their tailored solutions ensure that clients can move to the next phase of their studies more rapidly, raising funds and achieving successful exits.

Navigating the FDA certificate medical device approval landscape for medical products is crucial for clinical research professionals. Understanding the distinct pathways available—each tailored for different product types and associated risks—can significantly impact market readiness.
The primary pathways include:
Premarket Notification (510(k)): This route is utilized for products demonstrating substantial equivalence to an already marketed item. It requires evidence that the new device is as safe and effective as its predecessor. In 2022, 85% of 510(k) applications received a Substantially Equivalent decision, underscoring its effectiveness in facilitating market entry.
Premarket Approval (PMA): The PMA route is the most stringent, mandated for Class III products. It necessitates a comprehensive review of clinical data to ensure safety and efficacy. On average, a PMA decision takes approximately 449 days, reflecting the thoroughness of this process.
De Novo Classification: This pathway is designed for new products categorized as low to moderate risk without a predicate. It simplifies the authorization process, enabling faster market access while maintaining safety standards.
Humanitarian Device Exemption (HDE): Tailored for products intended to treat conditions affecting fewer than 8,000 individuals annually, the HDE pathway requires less clinical data than the PMA, thus facilitating access to innovative treatments for rare conditions.
Comprehending these pathways is essential for selecting the most appropriate route for your device to secure the FDA certificate medical device, potentially accelerating the endorsement timeline and enhancing market readiness. Notably, '98% of accelerated approvals and 96% of breakthrough therapy requests received priority review,' emphasizing the importance of strategic planning in navigating the FDA approval landscape. Additionally, leveraging comprehensive clinical trial management services, such as those offered by bioaccess®, can significantly enhance the efficiency and success of your clinical studies—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—particularly in the Latin American context.

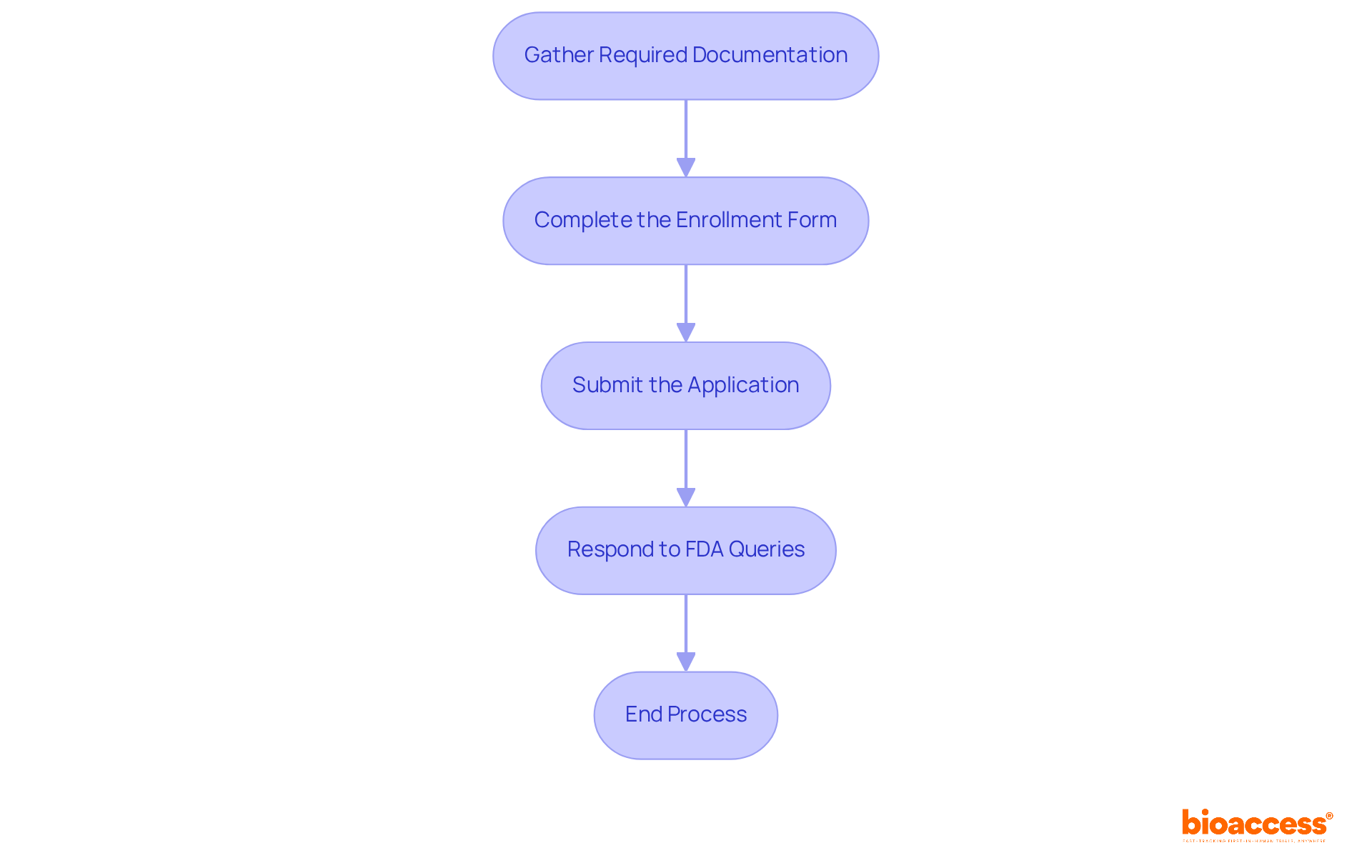
To effectively prepare and submit your FDA application, it is essential to adhere to the following steps:
Gather Required Documentation: Depending on your chosen regulatory pathway, compile essential documents, including device description and intended use, labeling and promotional materials, clinical data (if applicable), manufacturing information, and risk analysis along with mitigation strategies.
Complete the Enrollment Form: Accurately fill out the appropriate enrollment form for your selected pathway (e.g., 510(k), PMA). Ensure all sections are thoroughly completed to avoid delays in the process of obtaining the FDA certificate for medical devices.
Submit the Application: Utilize the FDA's electronic submission system (eSTAR) for your application. Confirm that all documents meet the required format and that applicable fees have been paid.
Respond to FDA Queries: After submission, be prepared to address any questions or requests for additional information from the FDA. Timely and comprehensive responses are crucial, as 67% of 510(k) submissions in recent years resulted in requests for additional information, which can prolong the review process.
In addition to these steps, consider leveraging comprehensive clinical trial management services, such as those offered by Bioaccess, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. These services can streamline your process and enhance your submission's success rate. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, can provide valuable insights and guidance throughout this process. Real-world examples highlight the importance of meticulous documentation: in 2024, 74% of novel drug approvals were cleared on the first review, underscoring the benefits of thorough preparation. Furthermore, the FDA's performance metrics indicate that 85% of 510(k) submissions received a Substantially Equivalent decision, emphasizing the importance of complying with documentation requirements. By following these steps and utilizing expert services, you can enhance your chances of a successful submission for an FDA certificate for medical devices.
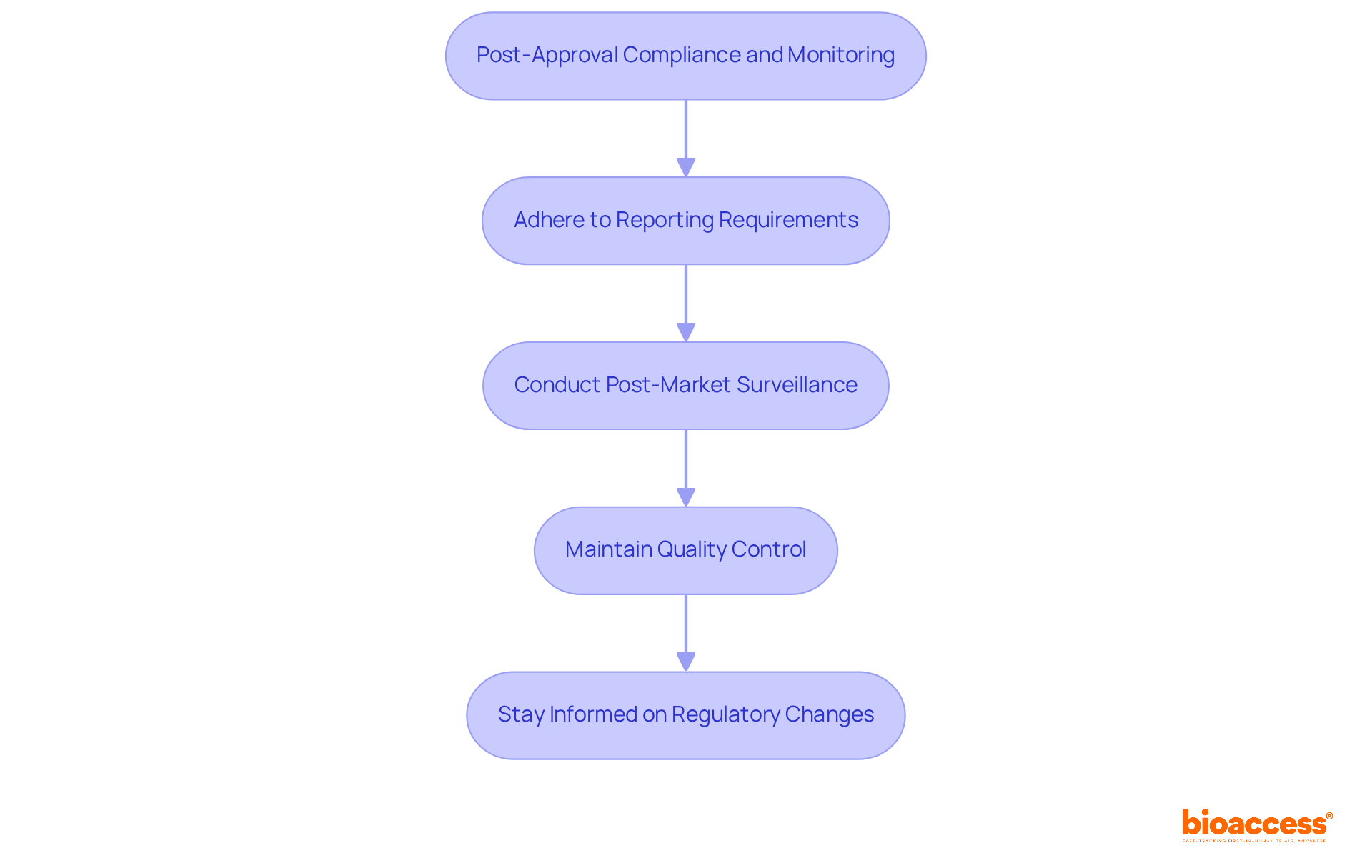
Once your medical device is approved, navigating post-approval compliance and monitoring is essential:
Adhere to Reporting Requirements: You must report any adverse events or equipment defects to the FDA, which includes establishing a robust system for tracking and reporting these incidents. In 2022, the FDA received approximately 18,800 submissions, highlighting the volume of reports that manufacturers must manage.
Conduct Post-Market Surveillance: Depending on the classification of the product, particularly for Class III categories, conducting post-market studies is crucial to monitor its performance and safety in real-world settings. The FDA emphasizes that effective post-market surveillance can significantly enhance patient safety and product quality for any medical device with an FDA certificate.
Maintain Quality Control: Ensure that your manufacturing processes consistently meet FDA standards. Routine evaluations and quality assessments are essential for ensuring compliance, as the FDA supervises over 6,700 medical product categories, requiring strict quality management practices.
Stay Informed on Regulatory Changes: The regulatory landscape is dynamic, so it’s imperative to stay updated on new guidelines or requirements from the FDA that may affect your product. In 2022, the FDA published or updated 57 guidance documents to align industry practices with regulatory expectations.
By diligently following these steps, you can ensure that your device remains compliant and secures the FDA certificate medical device, allowing it to continue delivering safe and effective solutions for patients.
Securing an FDA certificate for medical devices is a multifaceted process that demands a comprehensive understanding of regulatory pathways and compliance measures. This journey commences with the identification of your device's classification and the specific requirements linked to each risk category. By adhering to the FDA's guidelines and utilizing available resources, manufacturers can efficiently navigate the complexities of approval, thereby minimizing delays and maximizing the likelihood of successful market entry.
Key insights highlight the significance of selecting the appropriate regulatory pathway—such as Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo Classification—each tailored to distinct product types and risk levels. Proper documentation and timely responses to FDA inquiries are vital for avoiding setbacks during the review process. Additionally, post-approval compliance, which includes reporting requirements and ongoing surveillance, guarantees that devices continue to meet safety standards after market introduction.
Ultimately, the importance of comprehending and effectively managing the FDA approval process cannot be overstated. By taking proactive steps and leveraging expert services, manufacturers not only bolster their chances of securing an FDA certificate but also contribute to the safety and efficacy of medical devices in the healthcare landscape. Embracing these strategies empowers innovators to deliver groundbreaking solutions to patients in need, fostering advancements in medical technology that can significantly enhance lives.
What is the role of the FDA in medical device approval?
The FDA oversees the certification of medical devices, ensuring they meet safety and effectiveness standards before entering the market.
How are medical devices categorized by the FDA?
Medical devices are categorized into three groups based on their risk levels: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What are the regulatory requirements for each class of medical devices?
Class I products often qualify for exempt status, Class II products typically require a pre-market notification (PMN) application, and Class III products must undergo a more stringent pre-market authorization (PMA) process.
What can happen if a medical device fails to comply with FDA guidelines?
Failure to comply with FDA guidelines can lead to significant delays or outright rejections in the validation process.
Why have many preliminary clinical tests moved outside the United States?
Up to 75% of preliminary clinical testing has moved outside the U.S. due to prolonged regulatory timelines associated with FDA approvals.
What is the average time to receive a decision on FDA 510(k) applications?
The average time to issue a decision on FDA 510(k) applications is approximately 147 days, but this can extend further due to requests for additional information.
How can companies navigate the FDA approval process effectively?
Companies can utilize the FDA's resources, including their website and guidance documents, and maintain timely communication with the FDA to prevent obstacles in the approval process.
How can partnering with bioaccess® benefit companies in the clinical trial process?
Partnering with bioaccess®, a leading CRO in Latin America, can enhance the efficiency of clinical trials by providing expertise in regulatory navigation, clinical research site activation, patient recruitment, and trial data delivery, allowing clients to move to the next phase of their studies more rapidly.