


This article outlines strategies for reducing the timeline for medical device registration in Peru, emphasizing the necessity of understanding the regulatory landscape and engaging with local experts. By familiarizing oneself with the classification system and recent reforms, along with strategic planning and collaboration with specialists, stakeholders can significantly expedite the approval process. This approach not only streamlines operations but also facilitates faster market entry for medical products, underscoring the critical role of expert engagement in navigating the complexities of the Medtech landscape.
Navigating the medical device registration landscape in Peru presents both opportunities and challenges for manufacturers eager to enter this expanding market. A recent surge in the medical technology sector underscores the critical importance of understanding the regulatory framework established by DIGEMID.
As companies strive for expedited approvals, a pressing question emerges: how can stakeholders effectively streamline the registration process while overcoming common hurdles such as compliance complexity and documentation challenges?
This guide delves into strategies for reducing device registration timelines in Peru, offering insights that can significantly enhance market entry success.
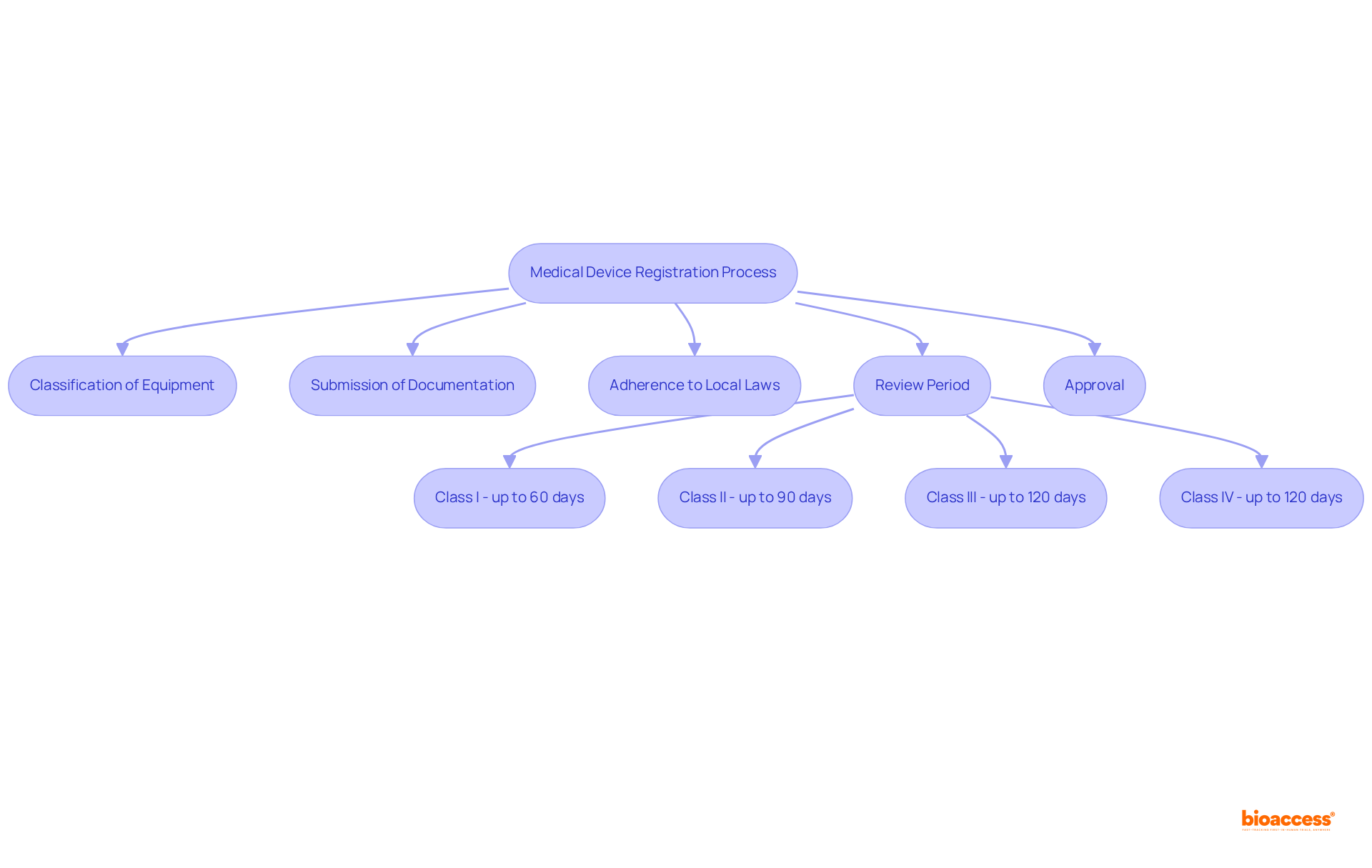
Successfully registering a medical product in Peru requires a comprehensive understanding of the regulatory framework established by the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID). This authority oversees the enrollment and authorization of medical equipment, ensuring compliance with safety and efficacy standards. The enrollment procedure typically encompasses several critical stages:
Familiarity with these requirements is essential for streamlining the enrollment process and minimizing potential challenges.
Medical equipment in Peru is categorized into four risk classes: I, II, III, and IV, with each classification influencing the approval timeline and documentation requirements. Class I items generally experience a faster registration process, while Classes II and III necessitate more comprehensive documentation and testing. For instance, the assessment period for Class I items can take up to 60 days, whereas Class II items may require up to 90 days, and Classes III and IV can extend to 120 days. Understanding these classifications is crucial for preparing the appropriate submissions tailored to your specific equipment type, ultimately facilitating a smoother path to market entry.
Recent reforms to Peru's approval framework have simplified and reduced approval durations, emphasizing the need for device registration timeline reduction Peru consulting to keep stakeholders well-informed. With the medical technology market in Peru witnessing a 1% growth in market value and a 9% increase in the number of medical items, grasping the registration process is more critical than ever. Moreover, bioaccess® has demonstrated its capability to achieve enrollment 50% faster than traditional markets, showcasing the effectiveness of navigating the compliance landscape in Peru. Their extensive services, including feasibility studies, investigator selection, trial setup, and project management, ensure that Medtech, Biopharma, and Radiopharma startups can advance their medical innovations more swiftly.
As Katherine Ruiz, an expert in Regulatory Affairs for medical equipment, asserts, "As the regulatory landscape changes, it is vital for stakeholders to remain informed and adjust their strategies accordingly." This perspective underscores the importance of continuous learning and adaptation in response to evolving regulations.
In conclusion, a thorough understanding of the enrollment process, incorporating the latest updates and market trends, is essential for successfully introducing medical products to the market in Peru. Collaborating with local experts and leveraging resources like bioaccess® can significantly enhance your chances of success.
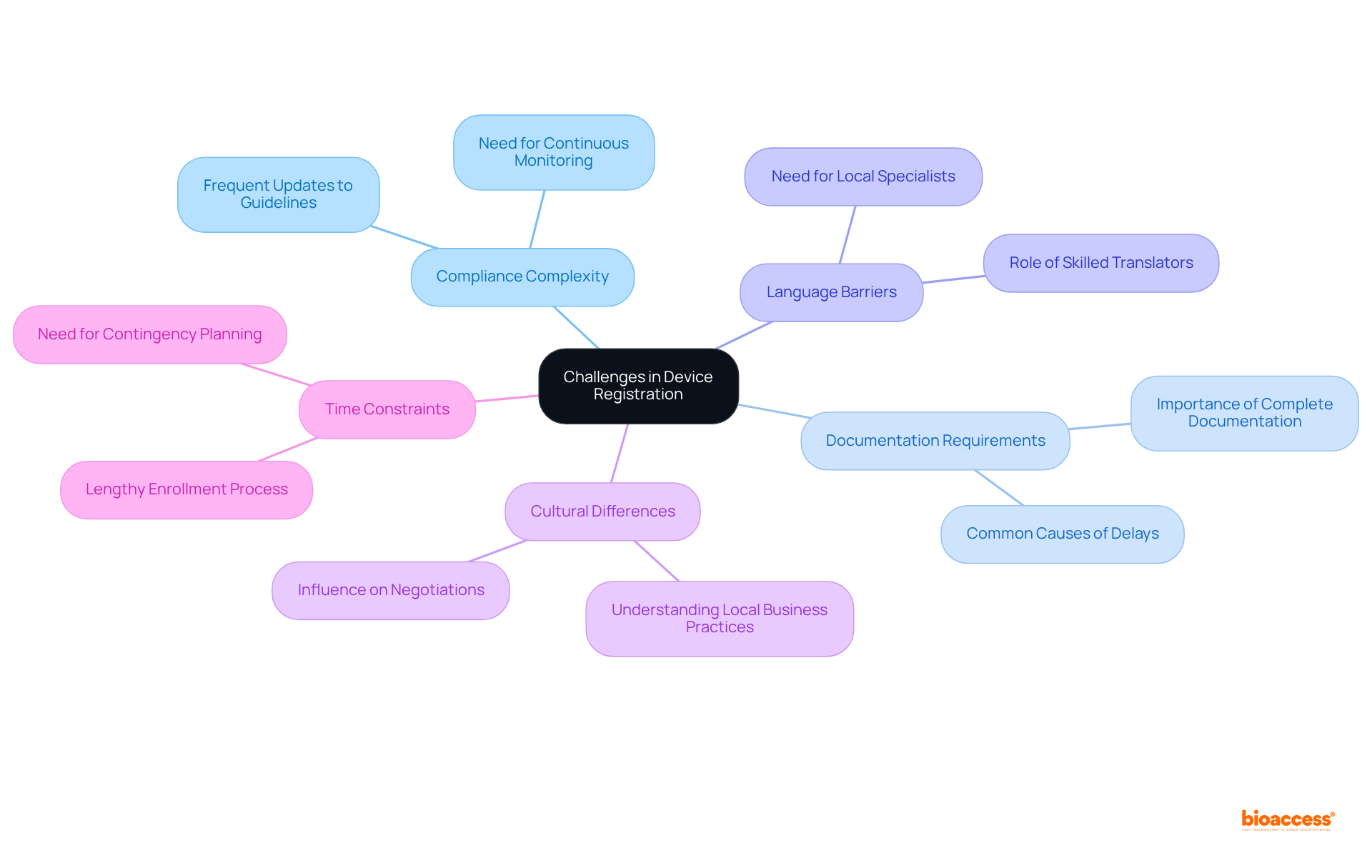
The challenges in the medical device registration process highlight the need for device registration timeline reduction Peru consulting to ensure timely approvals.
By proactively identifying and tackling these challenges, companies can enhance their strategies for device registration timeline reduction Peru consulting, leading to successful medical product approval and ultimately resulting in quicker market entry and better patient outcomes.
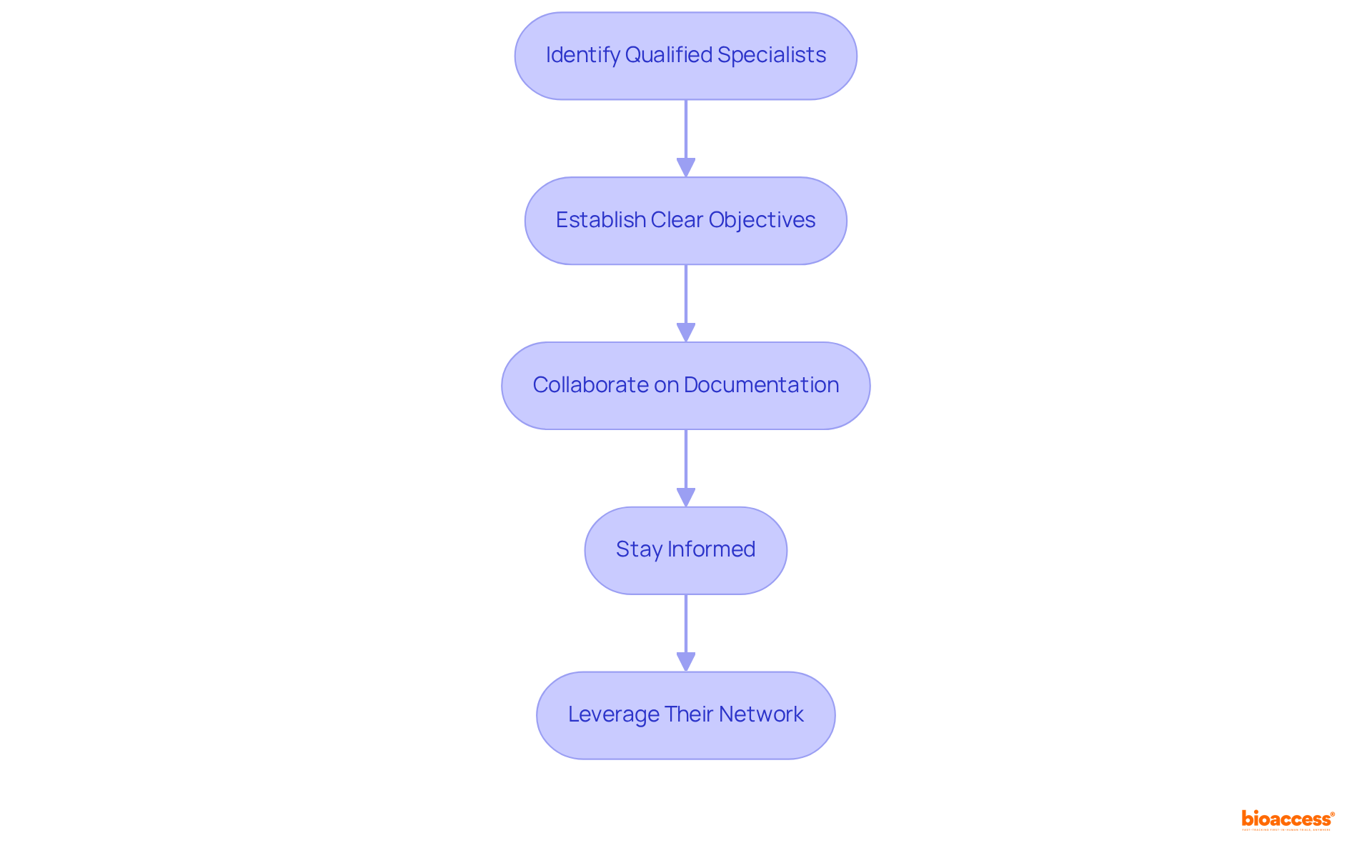
To navigate the complexities of medical device approval in Peru, collaborating with specialists such as Ana Criado, Director of Compliance at bioaccess, is immensely beneficial for device registration timeline reduction Peru consulting. With her extensive experience in biomedical engineering and compliance, she provides tailored guidance throughout the approval process. The following steps outline how to effectively engage with regulatory experts:
By aligning with compliance specialists, companies can enhance their understanding of the registration process and increase their chances of success. As Peru's medical equipment market is projected to grow, collaborating with consultants for device registration timeline reduction Peru consulting becomes increasingly essential for strategic market entry. Bioaccess offers comprehensive services, including trial setup, project management, and reporting, to assist companies in navigating these regulatory challenges.
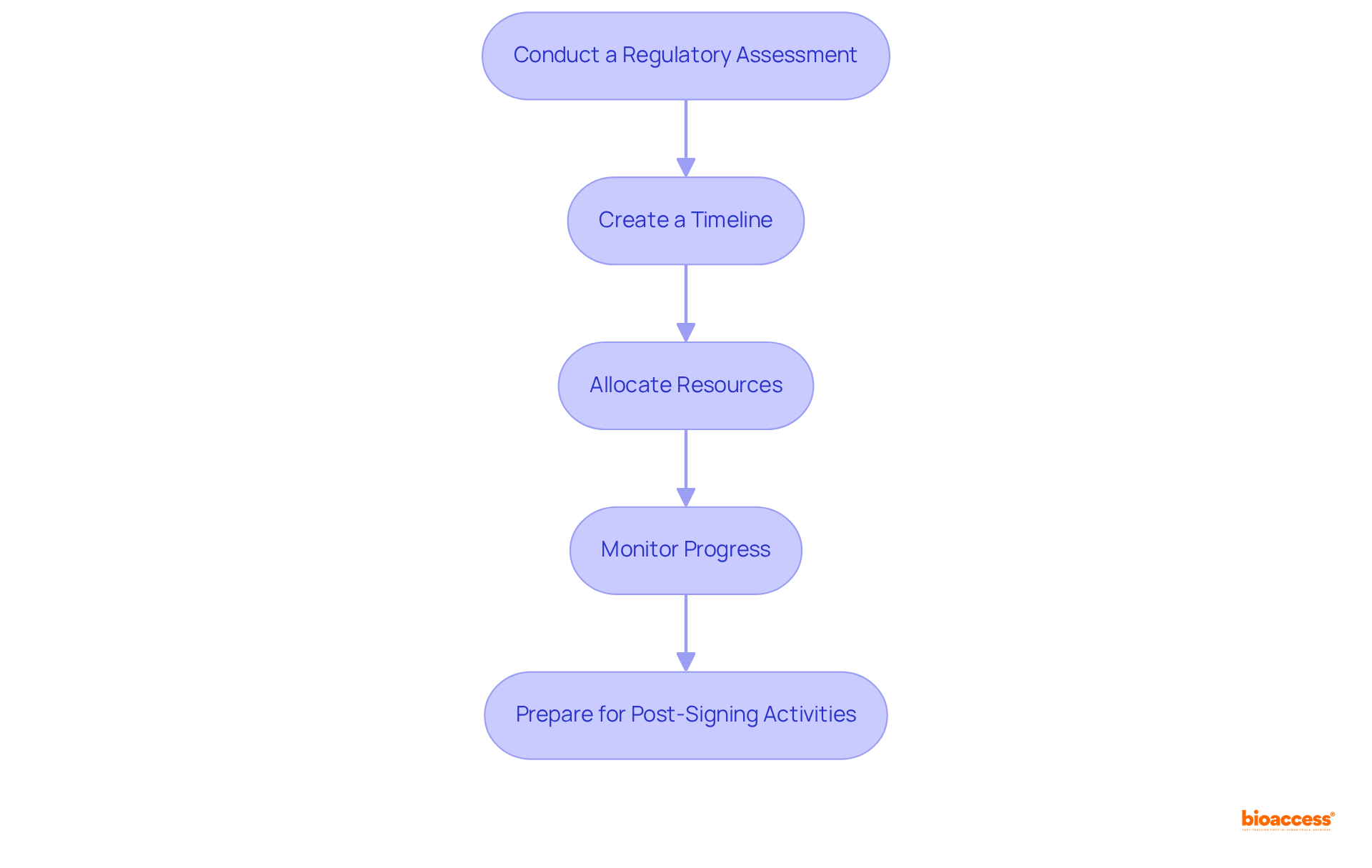
To guarantee an effective medical equipment approval process in Peru, creating a strategic plan is crucial. Here are the steps to create an effective registration strategy:
Conduct a Regulatory Assessment: Start by assessing the particular regulatory requirements for your equipment type. Familiarize yourself with the classification system, which categorizes devices into four risk classes (I, II, III, and IV), and understand the associated documentation and testing requirements mandated by DIGEMID.
Create a Timeline: Develop a detailed timeline that outlines each stage of the enrollment procedure, including deadlines for document preparation, submission, and follow-up. Ethical approvals in Peru can be achieved in just 4-6 weeks, making timely planning crucial.
Allocate Resources: Identify the necessary resources for the enrollment process, including personnel, budget, and external expertise. Ensure that you have the right support in place to navigate the complexities of the regulatory landscape effectively.
Monitor Progress: Regularly review the progress of your enrollment efforts against the established timeline. Adjust your plan as needed to address any delays or challenges that arise, ensuring that you remain on track to achieve faster enrollment, which is 50% quicker than traditional markets.
Prepare for Post-Signing Activities: Consider the steps needed after signing up, such as market entry strategies and adherence to post-market surveillance requirements. Engaging with local distributors can enhance your market presence and facilitate smoother operations.
By following these steps and maintaining a proactive approach, companies can achieve device registration timeline reduction through Peru consulting for their medical device registration process. Ultimately, this strategy reduces time to market and capitalizes on the anticipated growth of the medical device market, projected to reach US$2.30 billion by 2030.
Successfully navigating the medical device registration process in Peru is essential for companies aiming to introduce their products to this burgeoning market. A solid grasp of the regulatory landscape, including the classification of devices and the specific requirements set by DIGEMID, is crucial for minimizing delays and ensuring compliance. By understanding the nuances of the approval process, stakeholders can significantly streamline their timelines and enhance their chances of success.
Key insights from the article underscore the importance of identifying common challenges, such as:
Engaging with regulatory experts provides tailored guidance, enabling companies to effectively overcome these hurdles. Furthermore, developing a strategic plan that encompasses:
is vital for achieving efficient registration and facilitating quicker market entry.
In light of the anticipated growth of the medical device market in Peru, proactive engagement with local consultants and experts is more important than ever. By leveraging their knowledge and resources, companies can navigate the complexities of the registration process and position themselves for success in a competitive landscape. Embracing these strategies will ultimately lead to improved patient outcomes and a stronger foothold in the Peruvian medical technology market.
What is the regulatory authority responsible for medical device registration in Peru?
The regulatory authority responsible for medical device registration in Peru is the Dirección General de Medicamentos, Insumos y Drogas (DIGEMID).
What are the critical stages involved in the enrollment procedure for medical devices in Peru?
The critical stages involved in the enrollment procedure for medical devices in Peru include the classification of the equipment, submission of necessary documentation, and adherence to local laws.
How are medical devices classified in Peru, and why is this important?
Medical devices in Peru are classified into four risk classes: I, II, III, and IV. This classification is important because it influences the approval timeline and documentation requirements for each device type.
What is the typical registration timeline for different classes of medical devices in Peru?
The assessment period for Class I items can take up to 60 days, Class II items may require up to 90 days, and Classes III and IV can extend to 120 days.
How have recent reforms affected the medical device approval process in Peru?
Recent reforms have simplified and reduced approval durations for medical devices, making it essential for stakeholders to stay informed about the changes.
What growth trends are observed in the medical technology market in Peru?
The medical technology market in Peru has experienced a 1% growth in market value and a 9% increase in the number of medical items.
How does bioaccess® contribute to the medical device registration process in Peru?
Bioaccess® has shown the ability to achieve enrollment 50% faster than traditional markets by effectively navigating the compliance landscape and offering services like feasibility studies and project management.
Why is it important for stakeholders to stay informed about regulatory changes in Peru?
It is important for stakeholders to stay informed about regulatory changes in Peru to adjust their strategies accordingly and ensure successful market entry for their medical products.