


In the highly competitive biopharmaceutical industry, delays in clinical trials can lead to significant financial losses and postpone the delivery of vital treatments to patients. Facing recruitment challenges in traditional markets like the United States and Western Europe, sponsors are increasingly turning to Latin America. This region offers a distinct strategic advantage, consistently outperforming others in patient recruitment and retention.
This article explores the key factors driving Latin America's success. We will delve into the quantifiable data that shows faster enrollment and higher retention rates compared to the US and Europe. We will also examine the unique characteristics of the region's patient population, including its vast diversity and the prevalence of treatment-naïve individuals, which are ideal for producing clean and globally relevant clinical data. Furthermore, we will discuss the cultural dynamics, such as the high level of trust in the physician-patient relationship, and the significant economic and regulatory reforms—like Brazil's new streamlined approval process—that make Latin America a premier destination for clinical research. Finally, we will address the ethical considerations and operational hurdles, emphasizing the crucial role of expert local partners in navigating this promising landscape.
In the high-stakes world of pharmaceutical and medical device development, time is the most critical and unforgiving variable. The journey from a promising molecule to a market-ready therapy is a marathon fraught with obstacles, but none is more persistent or costly than patient recruitment. The statistics are a stark reminder of this systemic challenge: an estimated 85% of all clinical trials fail to recruit enough participants within their planned timelines, and a staggering 80% are ultimately delayed due to these recruitment shortfalls.1 Each delay is not merely a logistical inconvenience; it represents a significant financial hemorrhage, eroding patent life, delaying patient access to potentially life-saving treatments, and jeopardizing competitive standing in a crowded marketplace.
Faced with this persistent crisis, the biopharmaceutical industry has embarked on a strategic global migration. Over the past two decades, a clear trend has emerged: the offshoring of research and development activities away from the traditional, saturated markets of the United States and Western Europe toward emerging regions.2 This shift is driven by a search for greater efficiency, faster timelines, and access to more diverse patient populations. Studies have challenged the notion that this move is purely about cost, emphasizing instead the paramount importance of recruiting human subjects more rapidly to accelerate development timelines.2
Among the world's emerging research destinations, one region has consistently distinguished itself as a global leader: Latin America. The region's appeal extends far beyond simple cost efficiencies. It offers a demonstrably superior and more reliable environment for patient recruitment and retention, a claim supported by a powerful convergence of quantifiable performance metrics, unique population dynamics, a deeply ingrained culture of medical trust, and a rapidly modernizing regulatory landscape.2 This report provides an exhaustive, evidence-based analysis of the factors that underpin Latin America's recruitment advantage, demonstrating why the region has become the definitive strategic solution to one of the clinical research industry's most pressing and costly challenges.
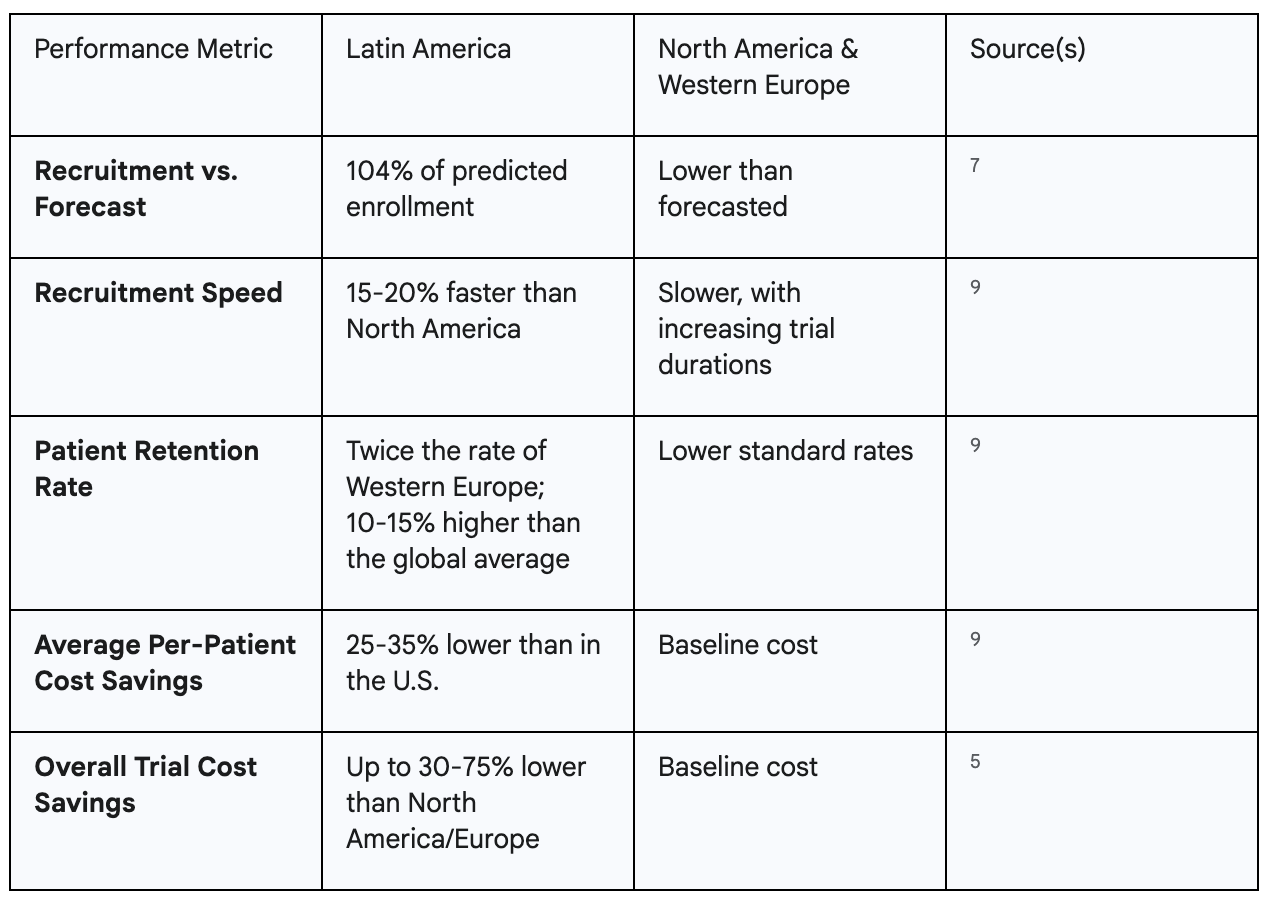
The assertion that Latin America offers a superior environment for clinical trial enrollment is not based on anecdote or marketing hyperbole; it is grounded in hard, quantifiable performance data. When key metrics for recruitment and retention are analyzed, a clear and consistent performance gap emerges between Latin America and the more traditional research hubs of North America and Western Europe.
The most compelling top-line metric comes from a comprehensive 2022 study published in PLOS ONE, which investigated the recruitment performance of clinical trial sites on a regional level. The findings were unequivocal. While recruitment performance was consistently lower than forecasted in Europe and North America, Latin America was the only region to exceed expectations, achieving an impressive 104% of its predicted recruitment targets.7 This single data point fundamentally reframes the global recruitment landscape, suggesting that timelines planned for Latin America are not only met but often surpassed, a level of reliability that is increasingly rare in developed nations.
This reliability translates directly into speed. Data from Brazil, one of the region's largest markets, shows that patient recruitment is typically 15% to 20% faster than in North America.9 For a sponsor, this acceleration is not an incremental gain; it represents a significant compression of the overall trial timeline, potentially shaving months off the development schedule and accelerating the path to regulatory submission and market launch.
Beyond the initial speed of enrollment, the integrity and efficiency of a trial depend heavily on patient retention—the ability to keep participants engaged and compliant throughout the entire study duration. High dropout rates can compromise statistical power, introduce bias, and trigger costly rescue recruitment efforts. Here again, Latin America demonstrates a profound advantage. Data indicates that patient retention rates in the region are generally twice those observed in Western Europe and are significantly greater than rates in the United States and Canada.10 More recent analyses from Brazil confirm this trend, showing retention rates that are
10% to 15% higher than global averages.9 This high level of retention is further validated by specific in-country case studies; for example, a partnership between GlobalCare Clinical Studies and bioaccess™ in Colombia successfully achieved a
95% retention rate in its ambulatory clinical study services.5
This positive performance in Latin America stands in stark contrast to the challenging trends observed in the West. Global data from industry-sponsored Phase III trials shows that the average recruitment duration has been steadily increasing over the last decade, rising from a median of 13 months in 2008-2011 to 18 months in 2016-2019.7 This trend has forced sponsors to increase the number of trial sites to enroll fewer participants per site, adding complexity and cost.11 In certain therapeutic areas, such as systemic lupus erythematosus, studies have noted that the enrollment of patients from North America has decreased over time, while proportions from other regions, like Eastern Europe, have increased to compensate.12
The combined effect of faster recruitment and higher retention in Latin America creates a powerful operational synergy. These are not two independent advantages but are deeply interconnected components of a virtuous cycle. First, by recruiting the target population more quickly, the overall trial timeline is shortened. This inherently reduces the window of time during which participants can be lost to follow-up for various reasons, naturally bolstering retention. Second, because retention rates are predictably higher, sponsors do not need to "over-recruit" as aggressively at the outset to compensate for anticipated dropouts. This makes the initial enrollment target smaller, more manageable, and faster to achieve. The result is a compounding effect where each metric reinforces the other, creating a more predictable, stable, and de-risked operational environment. For sponsors accustomed to the volatility and uncertainty of recruitment in the US and Europe, this predictability is one of Latin America's most valuable strategic assets.
While the quantitative metrics provide a compelling case, the true strategic depth of Latin America's advantage lies in the characteristics of its patient populations. The region offers access to participants who are not only more numerous and willing but are also more scientifically ideal for generating clean, robust, and globally relevant clinical data.
A critical factor in the quality of clinical trial data is the availability of "treatment-naïve" patients—individuals who have not undergone prior pharmacological treatment for the condition being studied.14 These participants are invaluable because their biological responses to an investigational therapy are not confounded by the lingering effects of previous medications, drug-drug interactions, or developed tolerances.15 This allows for a cleaner, more direct assessment of a new drug's safety and efficacy.
Latin America possesses large pools of treatment-naïve patients across numerous therapeutic areas, including infectious diseases, cardiovascular conditions, and oncology.10 This stands in sharp contrast to the situation in many high-income countries, where decades of widespread access to a vast array of pharmaceuticals mean that patients, particularly those with chronic conditions, have often tried multiple therapies.14 This "over-medicated" status makes them less ideal subjects for clinical trials, complicating recruitment and potentially muddying the resulting data. The ability to easily enroll treatment-naïve subjects in Latin America is a profound scientific advantage that directly enhances the integrity and interpretability of trial results.
The clinical research industry faces a well-documented and ethically troubling diversity crisis. For decades, clinical trials conducted in the United States and Europe have overwhelmingly enrolled White participants, creating a dangerous gap in knowledge about how new medicines affect minority populations.17 The statistics are alarming: in the U.S., Hispanics make up over 16% of the population but have historically accounted for as little as 1% of clinical trial participants.6 Similarly, studies have shown that Black and Hispanic women are significantly underrepresented in breast cancer trials relative to their disease burden.21
This homogeneity is no longer tenable. Regulatory bodies, led by the U.S. Food and Drug Administration (FDA), are increasingly mandating that sponsors develop and implement formal diversity action plans to ensure trial populations reflect the demographics of the patients who will ultimately use the product.24 Latin America, with its large, ethnically and genetically diverse population of over 600 million people, offers a direct and effective solution to this global imperative.5 Conducting trials in countries like Brazil, Mexico, Colombia, and Argentina allows sponsors to naturally enroll a broad spectrum of participants, generating more comprehensive data and ensuring the wide applicability of study results. This not only satisfies regulatory requirements but also leads to better, safer, and more effective medicines for the global population.
The success of recruitment in Latin America is also deeply rooted in a unique socio-cultural dynamic centered on the physician-patient relationship and the structure of regional healthcare systems. Numerous sources highlight the strong bond between patients and physicians in the region, a relationship often characterized by a high degree of trust, respect, and deference.6 This is not merely a matter of good bedside manner; it is a cultural norm that has a direct impact on trial participation. Unlike in some Western cultures where patient autonomy can lead to skepticism about research, patients in Latin America are often more inclined to accept their physician's recommendation to enroll in a clinical study, trusting that the doctor is acting in their best interest.10
This foundation of trust is powerfully combined with the realities of healthcare access. For many individuals, particularly those reliant on public healthcare systems, participating in a clinical trial provides a gateway to advanced medical care they might not otherwise receive.10 This includes access to novel investigational therapies, more frequent and thorough diagnostic testing, and one-on-one care from medical specialists and a dedicated research team—all typically at no cost to the participant.29 This alignment of interests, where the patient receives high-level care and the sponsor gains a willing participant, creates a powerful and mutually beneficial motivation for enrollment.
These factors—diversity, trust, and access—do not operate in isolation. They are interwoven in a way that creates a uniquely fertile ground for recruitment, especially among the very populations that are hardest to reach in developed nations. In the U.S. and Europe, a legacy of medical exploitation and a lack of cultural sensitivity have fostered deep-seated mistrust among many minority communities, which acts as a primary barrier to their participation in research.19 In Latin America, the dynamic is often inverted. The physician, a highly respected figure, acts as a trusted gatekeeper. When this trusted physician presents a clinical trial not just as research but as an opportunity for enhanced medical care, the proposition becomes highly compelling. This dynamic effectively dismantles the walls of skepticism that plague recruitment efforts elsewhere. Consequently, Latin America does not just offer a diverse population; it offers a
uniquely accessible diverse population, making it arguably the most effective global location for conducting inclusive and representative clinical research.
The powerful demographic and performance advantages of conducting clinical trials in Latin America are underpinned by an equally compelling strategic framework. This framework combines significant and sustainable cost efficiencies with a regulatory environment that is undergoing a revolutionary modernization, actively shedding its reputation for bureaucracy and embracing speed and predictability to attract global investment.
The business case for conducting research in Latin America is exceptionally strong. The most immediate and tangible benefit is a dramatic reduction in operational costs. Comprehensive analyses show that the expense of running a clinical trial in the region can be 30% to 75% lower than in North America or Western Europe.5 These are not marginal savings; they represent a fundamental shift in the financial feasibility of a research program, allowing sponsors to allocate resources more efficiently without compromising quality.
A closer look at specific markets reveals the sources of these savings. In Brazil, for example, the average price-per-patient in a medical device trial is 25% to 35% lower than in the United States. This is driven primarily by reduced operational expenses, with site fees and investigator payments being 20% to 30% lower than their U.S. counterparts.9 These efficiencies stem from a lower overall cost of living and labor, not from a reduction in the quality of care or infrastructure. In fact, many Latin American countries, like Colombia, boast high-quality healthcare systems with modern facilities and well-trained professionals, ensuring that studies are conducted under rigorous standards.13
The financial appeal is further enhanced by attractive government incentives designed to stimulate R&D investment. Colombia, for instance, offers a suite of powerful tax benefits for innovation projects, including a 100% tax deduction, a 25% tax discount, and a 50% future tax credit.5 When combined, these direct cost savings and favorable tax policies create a financial environment that is difficult for sponsors to ignore.
For years, the primary deterrent for many sponsors considering Latin America was the perception—and often, the reality—of slow, unpredictable, and bureaucratic regulatory processes.27 This historical challenge, however, is rapidly becoming obsolete as key countries in the region undertake ambitious and transformative regulatory reforms.
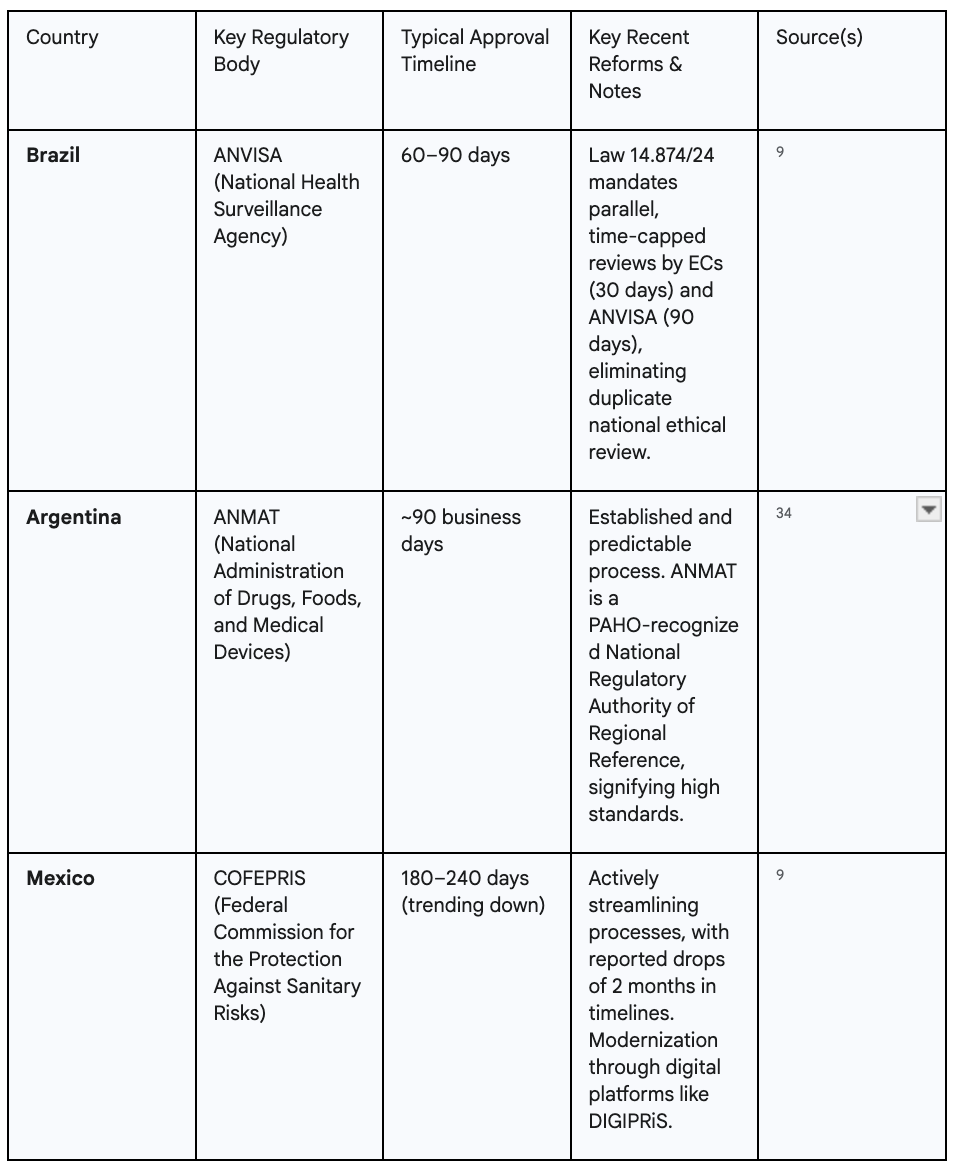
The most significant development is Brazil's landmark Law 14.874/24, enacted in 2024. This legislation has fundamentally revolutionized the country's clinical trial approval process. It eliminated a redundant, sequential review system, replacing it with a streamlined, parallel process where local Ethics Committees (ECs) and the national regulatory agency, ANVISA, review submissions simultaneously. Crucially, the law introduced hard, predictable timelines: EC reviews are now capped at 30 business days, and ANVISA's review is limited to 90 business days. This has slashed the overall approval time from a year or more to as little as 60 to 90 days, transforming Brazil into one of the most efficient regulatory environments in the world.9
This momentum is not confined to Brazil. Argentina's regulatory body, ANMAT—recognized by the Pan American Health Organization (PAHO) as a Regional Reference Authority—operates with an official clinical trial approval timeline of 90 business days.34 In Mexico, the national agency COFEPRIS is actively working to streamline its processes, with industry reports noting a drop of two months in approval times as a result of these efforts.36 The implementation of digital submission platforms like DIGIPRiS is further modernizing and accelerating review cycles.38
This wave of regulatory modernization acts as a powerful catalyst, fundamentally altering the strategic calculation for sponsors. The core advantages of Latin America—unparalleled patient access and lower costs—have been present for years. However, the risk of long, unpredictable regulatory delays often acted as a counterbalance, deterring investment. By systematically dismantling this key barrier, governments across the region are effectively unlocking Latin America's full potential. This is not an incremental improvement but a paradigm shift. It creates a unique and time-sensitive window of opportunity for sponsors. Those who act now, guided by expert partners who understand these new frameworks, can capitalize on the powerful combination of long-standing demographic advantages and brand-new regulatory efficiency, gaining a significant competitive edge before this new reality becomes common knowledge and the market grows more saturated.
Realizing the immense strategic potential of Latin America requires more than a simple acknowledgment of the opportunity. It demands a sophisticated, ethical, and expert-led approach to navigate the region's unique operational challenges and profound ethical responsibilities. The very factors that make the region so attractive—its diverse populations and different healthcare structures—also necessitate a higher degree of diligence and cultural competence.
A credible approach to research in Latin America must proactively engage with the complex ethical considerations inherent in conducting trials in the Global South. Academic analysis, most notably by researcher Manuela Fernández Pinto, has identified a potential "double disadvantage" for participants in the region.26 The first disadvantage stems from a historical lack of research focused on these populations, leading to gaps in understanding disease presentation and treatment response. The second, more direct disadvantage arises when these patients participate in trials for therapies that are primarily designed to meet the needs and commercial markets of the "Global North," which may not be optimally suited to their local health context or accessible to them post-approval.14
This ethical critique is not a reason to disengage from the region. On the contrary, it underscores the profound responsibility of sponsors and their CRO partners to conduct research that is truly beneficial to all stakeholders. It mandates a rigorously patient-centric approach that goes beyond mere compliance. This includes ensuring a robust and culturally sensitive informed consent process, especially given that some patients may have limited literacy or place immense trust in their physicians.10 It means designing trials that, whenever possible, address local health priorities and actively working to create pathways for post-trial access to successful therapies. Partnering with a responsible, ethically-minded CRO is the primary mechanism for mitigating these risks and ensuring that a clinical trial is a "win-win," advancing global science while providing tangible benefits to the participants and communities who make it possible.15
Alongside the ethical landscape, sponsors must navigate a series of practical and operational challenges. While the region boasts many high-quality research centers, there can be significant variation in site infrastructure, particularly regarding capabilities for electronic data capture (EDC) and other advanced technologies.30 The logistics of importing and exporting clinical trial materials—including investigational products, lab kits, and equipment—can be complex, with each country having specific customs, licensing, and documentation requirements that can cause delays if not managed proactively.33
Furthermore, all patient-facing materials, from informed consent forms to recruitment advertisements, must be carefully adapted to be linguistically accurate and culturally resonant.10 A direct translation is rarely sufficient; materials must be crafted in simple, understandable language that respects local norms and values to be effective. These challenges are not insurmountable barriers, but they are significant complexities that underscore the absolute necessity of having on-the-ground, specialized expertise. A successful trial hinges on a partner who possesses an intimate understanding of the local ecosystem—one who can perform rigorous site selection 40, provide essential support to local investigators 30, and develop patient engagement strategies that are precisely tailored to the culture.43
This is where the value of a specialized Contract Research Organization becomes indispensable. A CRO with deep roots in Latin America does far more than simply execute operational tasks. It functions as a strategic enabler that de-risks the entire clinical research venture. Such a partner provides up-to-the-minute regulatory intelligence, navigating the nuances of the new, faster approval pathways to prevent missteps.2 It serves as an ethical steward, ensuring that trial conduct meets the highest global standards while respecting local contexts. It manages the intricate web of logistics, from import permits to supply chain management, ensuring the trial runs smoothly and on schedule.16 Most importantly, it leverages its extensive local networks of hospitals, investigators, and community organizations to maximize the inherent recruitment and retention advantages of the region, transforming potential into performance.43
The evidence is clear and multifaceted. Latin America is no longer an "emerging" or "alternative" region for clinical research; it is a premier global destination that offers a proven, quantifiable advantage in the critical metrics of patient recruitment and retention. This superiority is driven by a powerful trifecta of factors: superior performance metrics that demonstrate unmatched speed and reliability; scientifically ideal patient populations that are diverse, engaged, and treatment-naïve; and a strategic landscape defined by compelling economics and a new era of regulatory efficiency.
This immense potential, however, is not unlocked by simply offshoring a trial protocol. It is realized through a thoughtful, ethical, and expert-led partnership. The cultural, logistical, and regulatory complexities of the region are real, but they are entirely navigable with a guide who possesses deep local knowledge and a commitment to responsible research. Choosing the right partner is the key to transforming Latin America's strategic advantages into a successful clinical trial that meets its timelines, stays on budget, and generates high-quality, globally relevant data.
The data is clear. The opportunity is now. To leverage the full strategic advantage of Latin America for your next clinical trial and ensure it is conducted efficiently, ethically, and successfully, partner with the experts who call this region home. Contact Bioaccessla today to start the conversation.
1. Why is patient recruitment faster in Latin America?
Patient recruitment in Latin America is faster due to a combination of factors. The region has a large, diverse, and often treatment-naïve population, meaning there is a larger pool of eligible participants for a wide range of studies.10 Culturally, there is a high degree of trust in the physician-patient relationship, making patients more receptive to participating in trials recommended by their doctors.6 Finally, for many, clinical trials offer access to advanced medical care and novel treatments that might otherwise be unavailable through public healthcare systems, creating a strong incentive for enrollment.10
2. Is it significantly cheaper to conduct clinical trials in Latin America?
Yes, there are significant cost efficiencies. Overall operational costs for clinical trials in Latin America can be 30% to 75% lower than in the United States or Western Europe.5 These savings are driven by factors such as lower per-patient costs, reduced site management fees, and more affordable investigator payments.9 Additionally, some countries offer attractive R&D tax incentives that further enhance the financial benefits.5
3. Aren't regulatory approvals in Latin America notoriously slow?
While this was a significant challenge historically, the regulatory landscape is undergoing a major transformation. Brazil, for example, enacted a new law in 2024 that has cut approval timelines from over a year to as little as 60-90 days.9 Argentina maintains a predictable 90-business-day timeline, and Mexico is actively streamlining its processes.34 These reforms are making the region increasingly competitive and predictable for sponsors.
4. How does conducting trials in Latin America help solve the diversity problem in research?
The U.S. and Europe have long struggled with a lack of diversity in clinical trials, with minority groups being severely underrepresented.17 Latin America, with its ethnically and genetically diverse population of over 600 million people, provides a direct solution.5 Conducting trials in the region allows sponsors to naturally enroll a more representative patient population, which is now a key requirement from regulatory bodies like the FDA. This leads to more robust data and ensures new treatments are safe and effective for a wider demographic.24
5. What are the main challenges or risks of running a trial in Latin America?
The primary challenges are operational, logistical, and ethical. Sponsors must navigate complex import/export regulations for trial materials, which can cause delays if not managed by experts.33 Site infrastructure can vary, and patient-facing documents require careful cultural and linguistic adaptation.10 Ethically, sponsors must be mindful of the "double disadvantage," ensuring trials are beneficial to the local population and not just serving the needs of markets in the Global North.26 Overcoming these challenges requires a strong partnership with a specialized CRO that has deep local expertise.