

By Julio Martinez-Clark, CEO, bioaccess®
For MedTech startups, the first-in-human (FIH) clinical trial is the most critical milestone. It proves your technology works in people, which unlocks funding and paves the way for market approval. However, running these trials in the U.S. or E.U. is often slow, bureaucratic, and expensive.
This guide explains a faster, more efficient path. You will learn how to leverage a global strategy for your FIH trial, with a special focus on why Latin America is the ideal destination for many U.S.-based startups. We'll cover the specific advantages of different regions, share real-world success stories, and outline a proven method for accelerating your journey from the lab to the clinic.
If you're a MedTech founder, you know the script. You have a brilliant technology, you've raised seed funding, and you've proven your concept in the lab. Your goal is a successful acquisition by an industry leader like Boston Scientific or Medtronic.
But a huge challenge stands in your way: the first-in-human (FIH) clinical trial. This is the first time your device will be tested in people. This stage is often called the MedTech "valley of death." Promising innovations can stall here. The road to market can take 5-10 years and cost $10-$30 million before an exit is possible. All the while, investors are "breathing down your neck" for a return.
Running your FIH trial in the United States or the European Union presents major hurdles:
This is why the FIH trial is your most critical value inflection point. It's the moment your technology becomes validated human data. Success de-risks your company and unlocks the next, larger round of financing. With positive human data, you can tell investors, "it works. I need more money". The speed at which you reach this point determines your company's valuation and survival.
The solution isn't fighting these barriers, but going around them. For many innovators, conducting early-phase trials overseas is a brilliant strategic move. As bioaccess® CEO Julio G. Martinez-Clark says, "Latin America, in my opinion, is the hidden gem of the first-in-human world". This guide will show you why.
For startups with limited resources, time is everything. The old way of running trials no longer works. A new, global approach has emerged.
The strategy is simple: gather initial safety and efficacy data quickly. This is often done with a small group of patients, sometimes just "five patients" at a "single site overseas". This initial data is the key to unlocking conversations with the FDA and potential acquirers. It builds the case for a larger, pivotal study in the United States.
This has created a map of global clinical trial hotspots. Smart companies are looking to these regions for their early-phase work:
The landscape is always changing. Poland and the Czech Republic were once popular, but joining the E.U. made their regulations more complex. Now, the focus has shifted to non-E.U. countries like "the Balkans, Serbia, Kosovo, Montenegro," and even Central Asian nations like Kazakhstan.
This global shift is driven by regulatory arbitrage. The high barriers in the U.S. and the E.U. (with its "notoriously complex Medical Device Regulation") have created an opportunity for other nations to compete on speed and efficiency. Today's top MedTech founders are global strategists. They choose the jurisdiction that best fits their timeline and budget.
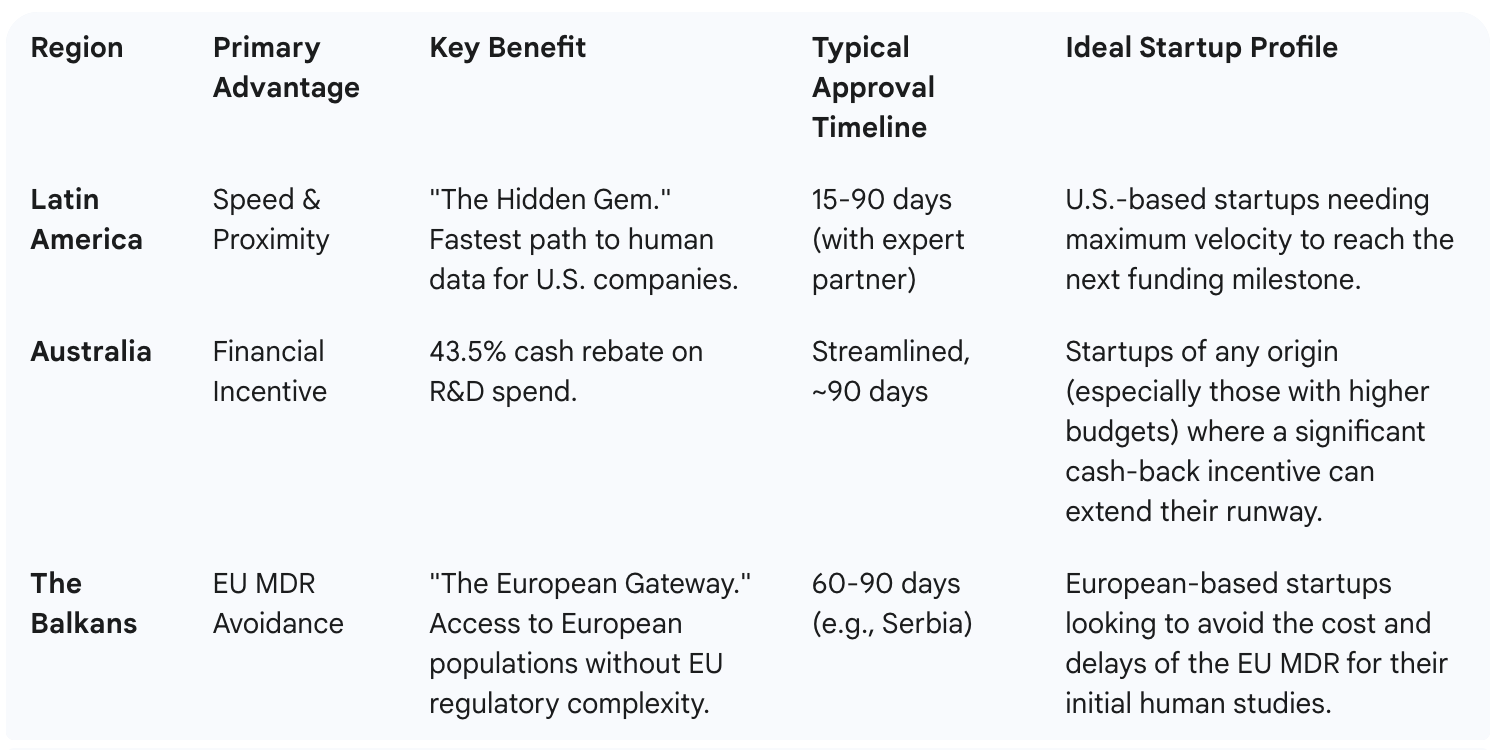
While many regions offer opportunities, Latin America provides a unique combination of speed, proximity, and expertise. It is the "hidden gem" for U.S.-based startups.
For U.S. companies, Latin America is the "natural" choice. The logistics are simple. A flight from Miami to Colombia, Panama, or the Dominican Republic is "under three hours".
This proximity is a major operational benefit. Implanting a new device often requires a "whole team of probably five people from the startup company... to fly... to get into the OR". Operating in a nearby, time-zone-aligned region means:
Latin America's biggest advantage is speed. In countries like "Panama, El Salvador, Chile, Paraguay, and the Dominican Republic," trial approvals are fast.
However, the key to unlocking this speed is having a partner who knows "who to talk to". An expert with a trusted local network can turn a months-long process into days. With 15 years of experience, bioaccess® has built these relationships. As Martinez-Clark notes, "On the first pass, we sometimes get [ethical] approvals in a record 15 days because we already know what to do".
Even Brazil, a country once known for "lengthy and complex regulatory approval processes," is changing. A new law is set to streamline its framework for medical device research. This development will make "Brazil one of the 'hotspots' of medical device research very soon".
The best location depends on your startup's specific needs. A true global partner can help you navigate the options.
Australia is a top destination for medical device and biopharma trials, thanks to a powerful financial incentive. The government offers a "very generous... tax rebate" for R&D.
Simply put: "whatever money you invest in your clinical trial in Australia, you are going to get 43.5 percent back [in check form]... from the government". This direct cash rebate can significantly extend your startup's runway. Combined with fast approvals and a "similar culture," Australia is a very attractive option.
For European startups, the challenge is avoiding the E.U.'s complex Medical Device Regulation (EU MDR). The solution is the Balkan states.
Countries like Serbia and Kosovo "are not yet part of the European Union" and offer a gateway to the continent without the red tape. These "sizable countries with streamlined regulations" are a competitive alternative. For instance, "in Serbia, you can get a trial approved in 60 to 90 days," which is a fast and efficient path to human data.
The world's most advanced innovators are using this global model to accelerate development.
Case Study 1: Axoft & The Future of Brain-Computer InterfaceAxoft, a "spinoff company from Harvard Labs," is developing a brain-computer interface (BCI) similar to Neuralink. Working with bioaccess®, Axoft gained trial approval in a top Panama hospital in a "record 60 days". This shows that even the most advanced technologies can find a faster path in Latin America.
Case Study 2: Newrotex & The New Speed RecordNewrotex, an "Oxford University spinoff developing breakthrough nerve regeneration technology out of silk," achieved an even faster result. The company received a "15-day ethical approval in Panama" for its FIH trial. The fact that spin-offs from Harvard and Oxford trust this model is a powerful endorsement.
Case Study 3: enVVeno Medical & The Path to U.S. Pivotal TrialsThis case shows the full strategic cycle. enVVeno Medical ran a study in Colombia for its VenoValve, a device for treating chronic venous insufficiency. The successful Phase 1 data allowed the company to start a larger "pivotal trial in the U.S." This proves that data from Latin America is a valid stepping stone to the U.S. market. The company's Chief Medical Officer, Dr. Marc Glickman, recently appeared on national news discussing this very condition.
Achieving these results requires a proven methodology that combines networks, technology, and a clear mission.
The biggest barrier for founders is often knowing "who to talk to". The bioaccess® network was built to solve this. It includes "more than 150 active clinical research sites globally" and is based on trusted relationships cultivated over 15 years. This ensures every project is matched with the right, pre-vetted investigators.
Human expertise is enhanced by technology. bioaccess® uses artificial intelligence to speed up processes that cause delays. AI tools are used to:
This work is driven by a mission to empower innovators. CEO Julio Martinez-Clark identified a "knowledge gap" in the industry about FIH trials.[1, 1] He started by writing articles and later launched the "Global Trial Accelerators™" podcast. With nearly 100 episodes, the podcast helps new founders "avoid those mistakes so they're more effective and they save money and time".
The hurdles in the U.S. and E.U. trial systems are real, but they don't have to slow you down. A strategic global approach offers a fast and efficient path to the human data you need to drive valuation and secure funding. With its unique blend of speed and proximity, Latin America is the "hidden gem" for this critical stage.
Success, however, requires the right partner. The bioaccess® method—combining a deep network, AI-powered tools, and an expert team—provides the guidance needed to navigate the global landscape.
Your breakthrough technology can't afford to wait. The path to your next value inflection point is faster than you think. Contact bioaccess® today for a confidential consultation to map your technology to the optimal regulatory pathway.