


The article identifies the foremost contract research organizations (CROs) pivotal to the success of clinical research. It underscores key players such as bioaccess®, IQVIA, and ICON plc, highlighting their strengths in:
These attributes collectively enhance the overall success of clinical trials, demonstrating the vital role these organizations play in the Medtech landscape. Understanding these strengths is essential for stakeholders aiming to navigate the complexities of clinical research effectively.
In an era where the race for groundbreaking medical advancements is more competitive than ever, the role of Contract Research Organizations (CROs) has become pivotal in shaping the future of clinical research. These specialized firms not only streamline the research process but also offer significant cost savings and faster patient recruitment—crucial elements for bringing innovative therapies to market.
However, with a plethora of options available, how can researchers identify the top CROs that will ensure their clinical trials succeed? This article delves into the leading CROs of 2025, highlighting their unique strengths and contributions to the evolving landscape of clinical research.
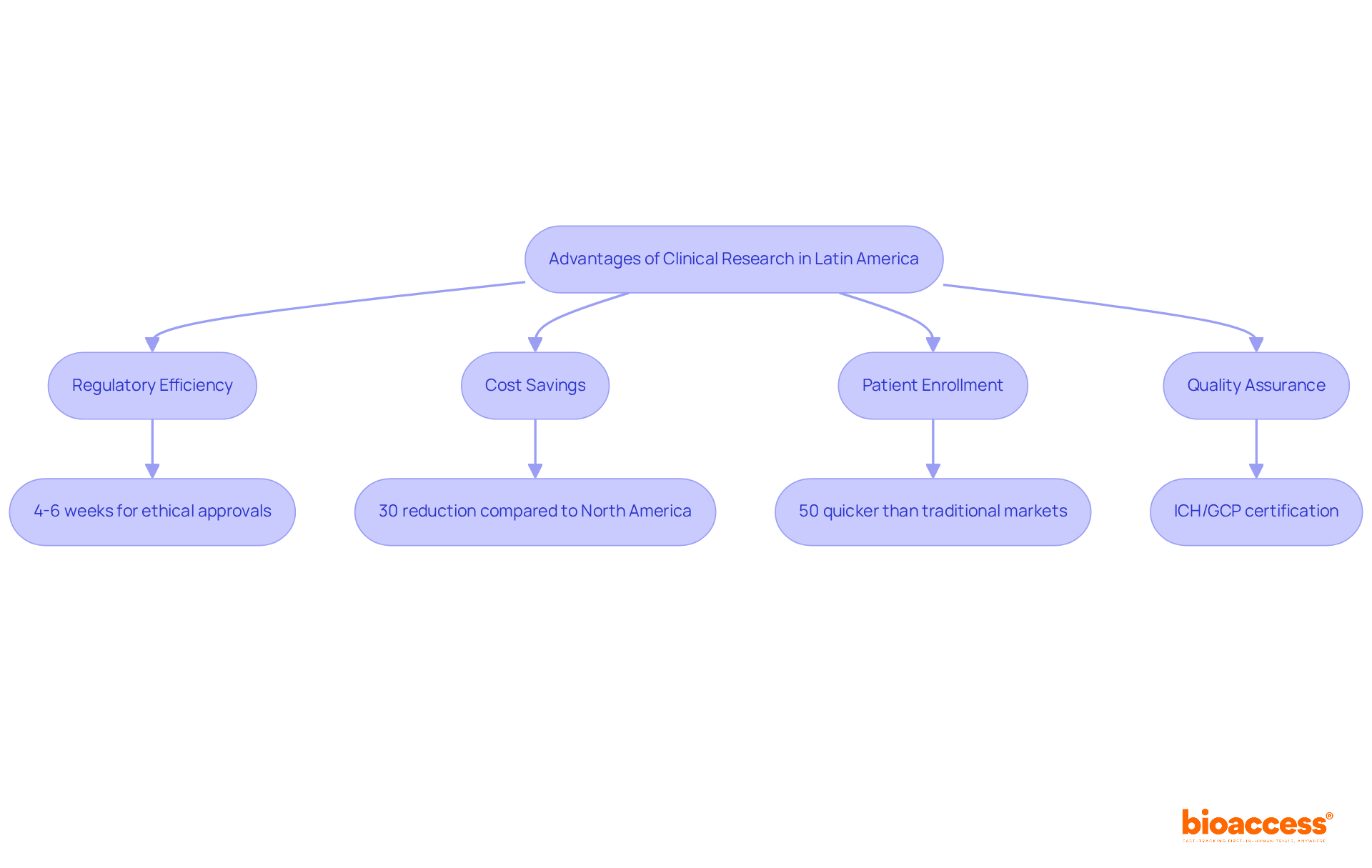
bioaccess® distinguishes itself within the research environment by leveraging the regulatory efficiency of Latin America and its diverse patient demographics. Ethical approvals are secured in an impressive 4-6 weeks, significantly faster than the global average of 48 days, while patient enrollment occurs at a pace 50% quicker than traditional markets. This efficiency translates into substantial cost savings, with estimates indicating reductions of over 30% compared to experiments conducted in North America or Western Europe. Moreover, investments in science, technology, and innovation projects in Colombia benefit from R&D tax incentives, including a 100% tax deduction and various financial grants. Colombia's healthcare system, ranked #22 by the World Health Organization, along with its hospitals recognized as among the best in Latin America, further enhances the quality of medical trials. Additionally, hospitals in Colombia must undergo a stringent ICH/GCP certification process to conduct medical studies, ensuring high standards of quality assurance.
With over 15 years of experience in early-stage trials, bioaccess® provides customized, high-quality services that empower Medtech, Biopharma, and Radiopharma innovators to accelerate their breakthroughs. Notably, partnerships have demonstrated the effectiveness of this approach, with collaborators reporting a 50% reduction in recruitment time and 95% retention rates in research studies. As Julio G. Martinez-Clark, CEO of a prominent organization, aptly states, 'Latin America is the hidden gem of the first-in-human world,' underscoring the region's potential for expediting medical studies and delivering innovative healthcare solutions to market swiftly.
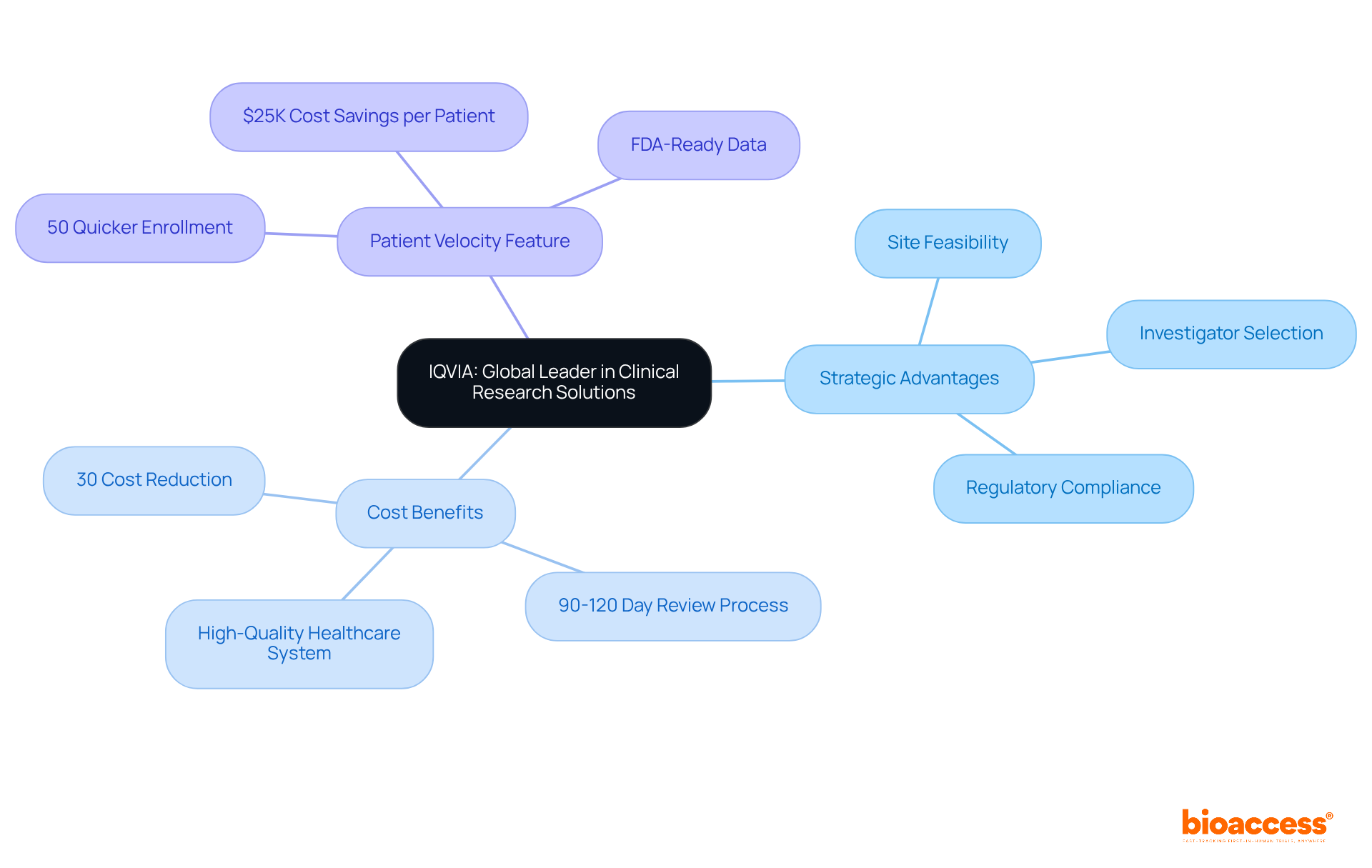
The company integrates advanced analytics, regulatory knowledge, and extensive clinical research services to provide comprehensive solutions throughout the medical device research lifecycle. Their focus on site feasibility, investigator selection, and regulatory compliance ensures that trials are set up efficiently and effectively.
With a strong presence in Colombia, the company capitalizes on the nation's competitive advantages, including:
Notably, the 'Patient Velocity' feature facilitates a 50% quicker enrollment of treatment-naive groups, resulting in substantial cost savings of $25K per patient with FDA-ready data. This strategic positioning empowers the organization to assist clients in navigating the complexities of research studies, establishing them as a preferred collaborator for numerous medical device firms aiming to accelerate their research and development initiatives.
To explore how bioaccess can enhance your study experience in Colombia, consider scheduling a meeting with their team.
ICON plc delivers a comprehensive suite of study management services, encompassing every aspect from study design to regulatory submission. With an unwavering focus on efficiency, ICON has achieved remarkable success rates in managing complex trials across various therapeutic areas, notably oncology, which constituted 37.12% of their revenue in 2024. Their commitment to quality is evident in their ability to secure ethical approvals in just 4-6 weeks, significantly accelerating the development process. This efficiency not only enhances the speed at which innovative treatments reach the market but also positions ICON as a reliable partner for organizations navigating the complexities of research.
By leveraging their extensive industry expertise and tailored solutions, ICON ensures that clients can effectively meet their specific needs and achieve favorable outcomes in their research endeavors. Furthermore, ICON was honored as the Best Contract Research Organisation (FSP) at the Scrip Awards in 2022, further cementing their reputation within the list of contract research organization in the industry.
With approximately 41,900 employees across 106 locations in 55 countries, ICON's scale and global reach empower them to support clients effectively. Their financial performance is also noteworthy, boasting a full-year adjusted EBITDA of $1,735.8 million, which represents 21.0% of revenue, alongside a net book-to-bill ratio of 1.20, indicating robust operational efficiency.
As the biopharmaceutical sector is poised for significant expansion, ICON is strategically positioned to capitalize on emerging opportunities in the research management services market, projected to reach USD 53,846.7 million by 2030. In comparison, biological access provides a thorough process for enhancing medical device evaluations, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. Their ability to enroll treatment-naive cardiology or neurology groups 50% faster than Western locations, combined with $25K savings per patient through FDA-ready data, establishes the organization as a competitive player in the research landscape.
![]()
The organization presents a comprehensive suite of services tailored to facilitate the management of studies, particularly in first-in-human medical device research within Colombia. Their expertise encompasses:
All designed to ensure a seamless process for biopharmaceutical companies. Notably, with an emphasis on accelerated patient recruitment, this organization can enroll treatment-naive cohorts 50% faster than Western sites, translating to significant cost savings of $25K per patient, all while providing FDA-ready data. Their collaboration with Caribbean Health Group positions Barranquilla as a leading hub for research studies in Latin America, a fact endorsed by Colombia's Minister of Health. The organization's unwavering commitment to innovation and patient-centric strategies establishes them as a vital partner for successful studies.
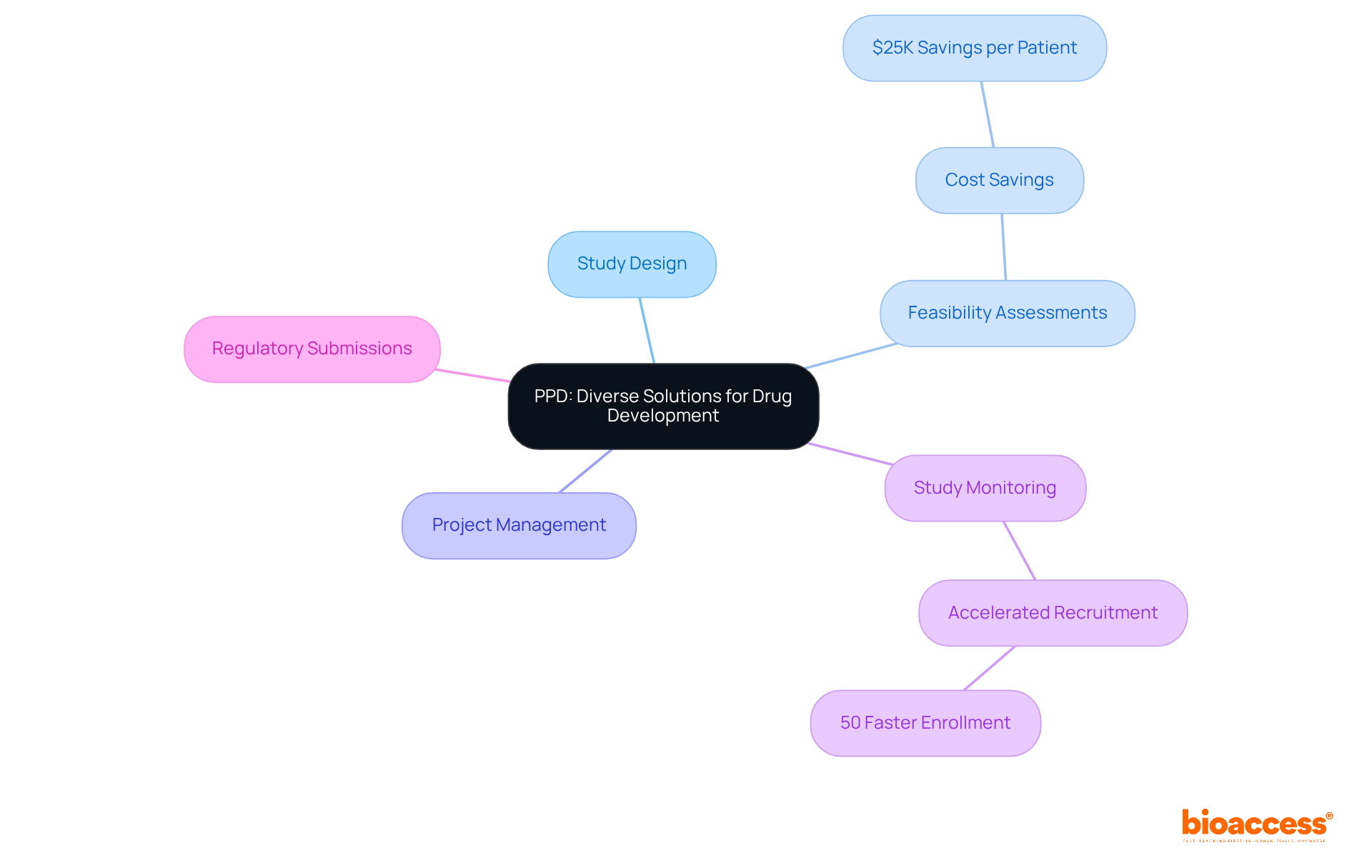
The company specializes in biopharmaceutical development, offering a comprehensive array of services that include:
Their deep understanding of the research environment allows them to provide customized solutions that effectively tackle the unique challenges faced by clients, particularly in LATAM. The partnership between Bioaccess and Beacon Launch Partners enhances their service offerings, ensuring quality and compliance throughout the research process. Their dedication to excellence results in:
Furthermore, the organization’s strategic focus on regulatory adherence and project oversight is crucial, enabling clients to navigate the complexities of research studies efficiently, thereby improving their prospects within the healthcare sector.
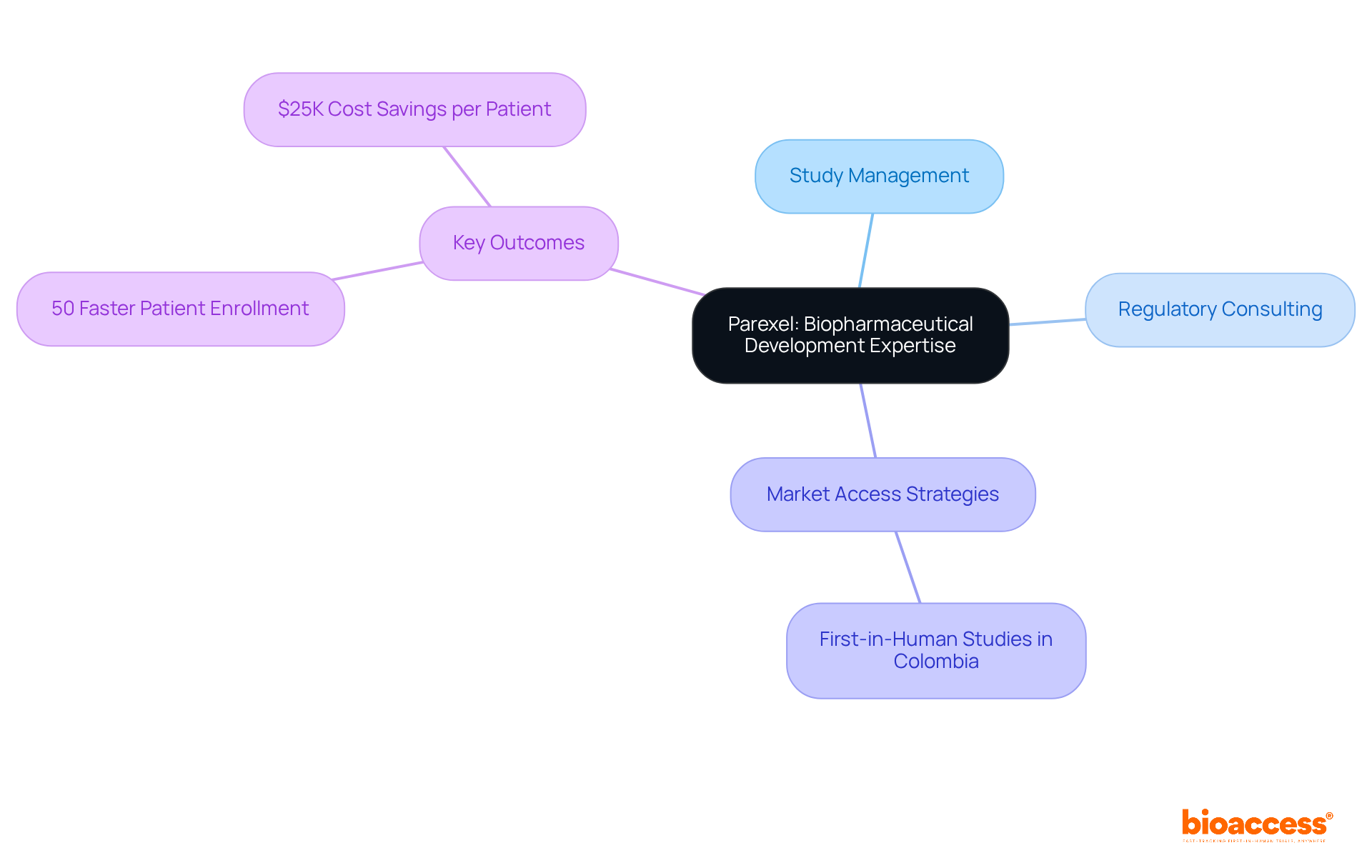
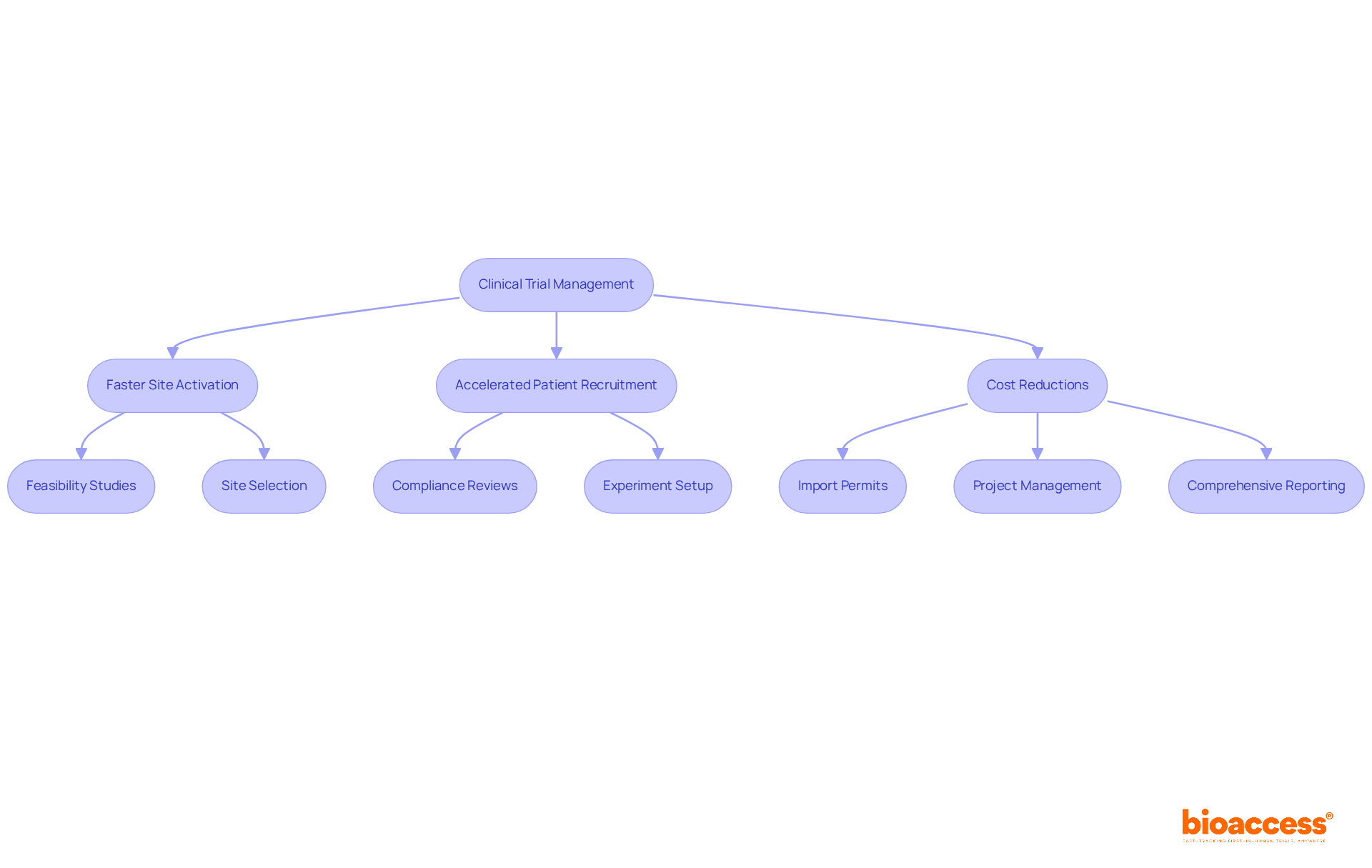
The company employs a comprehensive strategy for medical advancement and marketing, facilitating a seamless transition from research studies to product launch. This dual expertise ensures the delivery of end-to-end solutions that significantly enhance both efficiency and effectiveness. By aligning clinical and commercial strategies, the organization empowers clients to maximize their product potential, guaranteeing that innovative therapies reach the market swiftly and successfully. Their services include:
All designed to expedite regulatory approval and patient enrollment. Notably, the organization's commitment to these integrated solutions has been pivotal in achieving successful commercialization results, as evidenced by various case studies that illustrate improved market readiness and reduced time to market for their clients' products. Currently, the organization continues to enhance its solutions, adapting to the evolving landscape of research and commercialization, thereby reinforcing its position as a leader in the sector. With the capability to enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites and realizing $25K savings per patient with FDA-ready data, this organization is resolutely dedicated to delivering exceptional value to its clients.
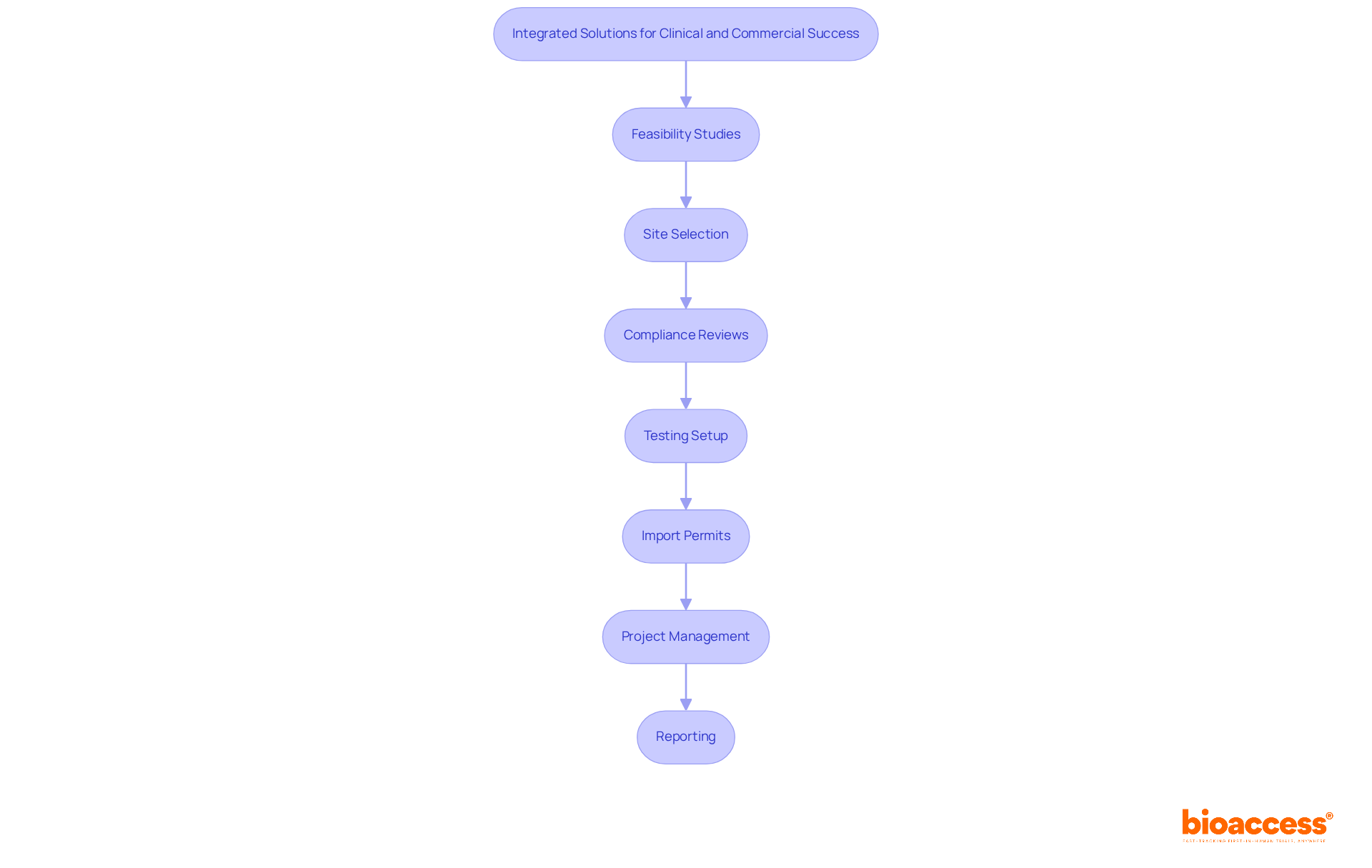
This company emerges as a leader in providing comprehensive clinical trial management services, particularly across Latin America, Eastern Europe, and Australia. With a steadfast commitment to accelerating site activation, the organization is capable of initiating studies in less than eight weeks, ensuring FDA/EMA/MDR-ready datasets with centralized monitoring. This rapid approach streamlines the regulatory process and enhances patient recruitment, allowing for treatment-naive cardiology or neurology cohorts to be enrolled 50% faster than traditional Western sites.
Furthermore, the platform delivers substantial cost reductions of $25K per patient through its FDA-ready data, eliminating the need for rework and delays. Their extensive service capabilities encompass:
As the study environment becomes increasingly competitive, their groundbreaking solutions and regulatory expertise position them as an essential ally for Medtech and biopharma startups striving for success in their evaluations. To explore how bioaccess can support your research needs, consider arranging a meeting with our team.
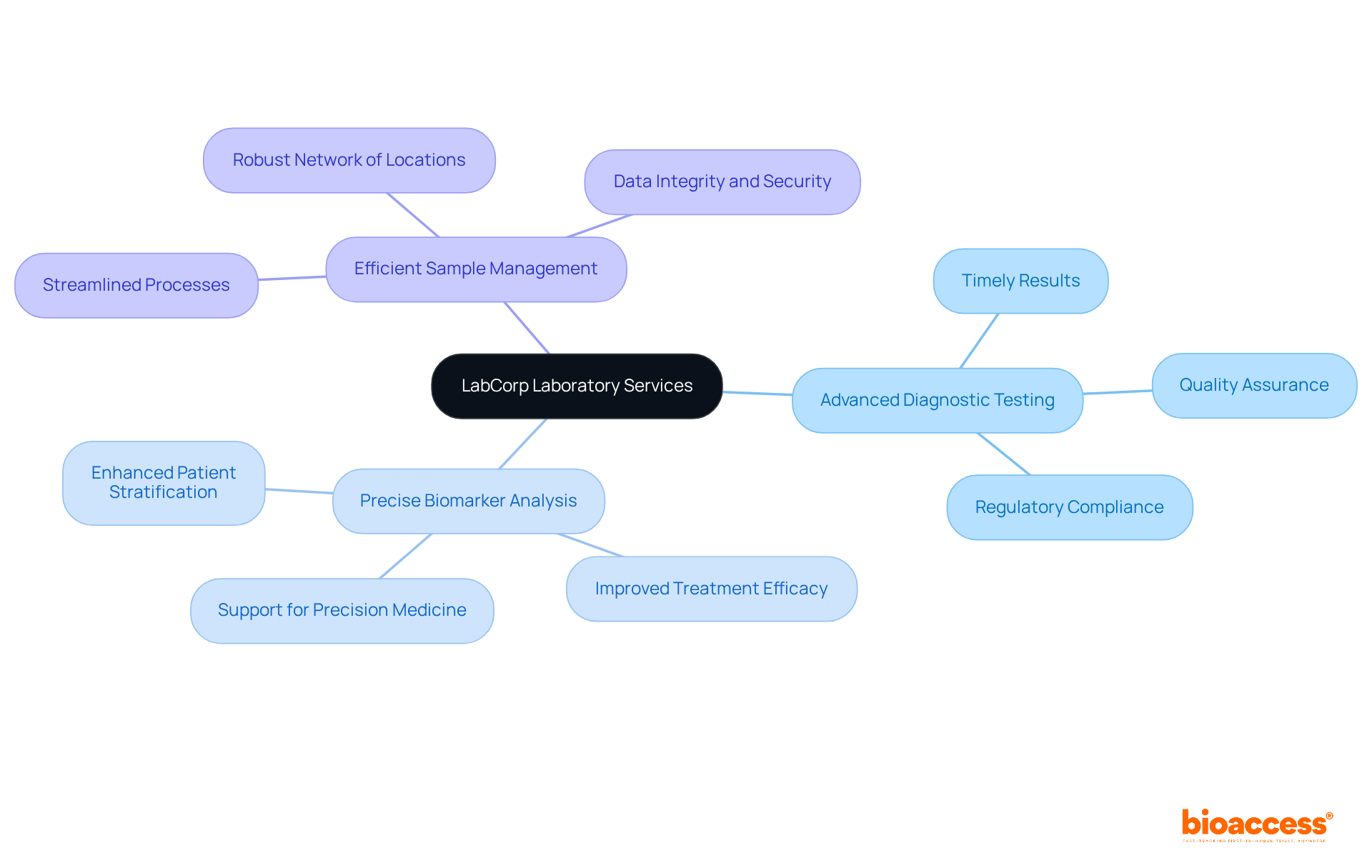
Laboratory Corporation of America (LabCorp) provides a comprehensive suite of laboratory services that are essential for the success of research studies. Their offerings encompass:
All designed to enhance outcomes. With a robust network of over 500 study locations across the U.S., LabCorp ensures timely and accurate results, which are critical for informed decision-making in medical research. Their unwavering commitment to quality and regulatory compliance positions LabCorp as a trusted partner for medical research organizations, facilitating successful diagnostic testing and improving the overall efficacy of health studies.
For research directors, understanding the intricate processes involved in studies—such as site feasibility, investigator selection, and project management—while recognizing these challenges can significantly bolster study success.
The company offers a robust suite of comprehensive management services designed to enhance the efficiency and effectiveness of drug development. Its capabilities encompass:
This ensures that clients receive high-quality results tailored to their specific needs. Notably, the organization facilitates 50% faster patient registration and achieves $25K savings with FDA-ready data, accelerating studies and establishing itself as a vital ally for startups facing regulatory challenges. As the sector evolves, the organization remains committed to expediting innovations and supporting clients in navigating early-stage study challenges, solidifying its position as a leader in advancing healthcare.

The platform employs cutting-edge technology to enhance clinical research procedures, delivering innovative solutions that significantly elevate efficiency and data quality. By emphasizing digital tools and data analytics, bioaccess facilitates real-time monitoring and informed decision-making, ensuring the seamless execution of studies. Their commitment to technology-driven solutions has led to substantial improvements in schedule timelines and participant engagement.
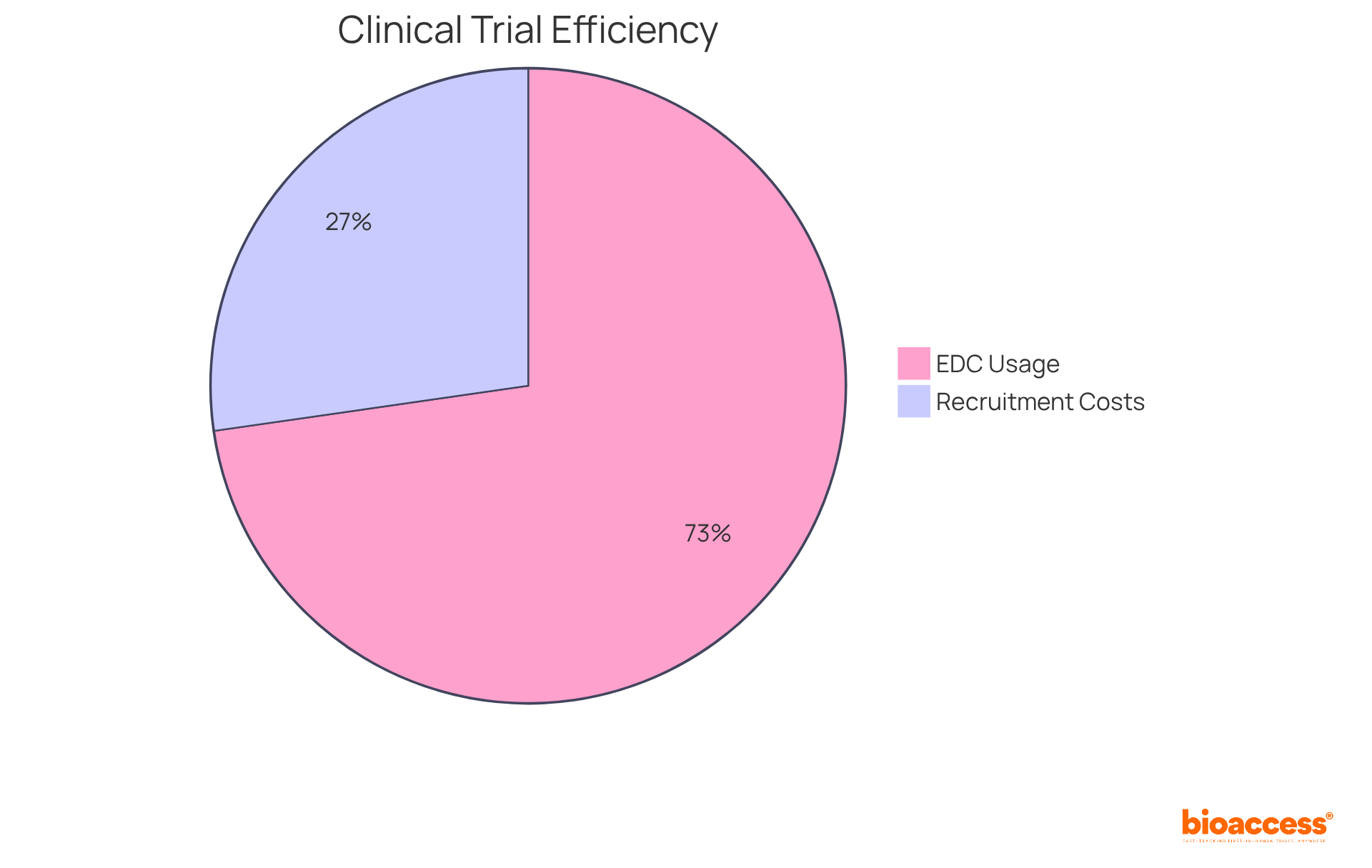
Notably, statistics reveal that nearly 80% of medical studies currently utilize electronic data capture systems, marking a pivotal shift towards digital integration. This transformation is crucial; in December 2020, 43% of surveyed research sites reported no use of digital tools, but by 2023, this figure decreased to 20%. The effective implementation of these technologies positions the organization at the forefront of the evolving healthcare landscape, underscoring their vital role in enhancing efficiency and effectiveness in medical studies.
Furthermore, with participant recruitment accounting for approximately 30% of overall costs in research studies, this technology-driven strategy not only boosts efficiency but also contributes to cost-effectiveness. In a competitive arena characterized by intense rivalry in the research technology sector, bioaccess stands out as a leader, showcasing the transformative potential of AI and ML in medical research. Their partnership with Caribbean Health Group aims to establish Barranquilla as a premier destination for clinical trials in Latin America, supported by Colombia's Minister of Health, thereby amplifying their impact in the region.
The landscape of clinical research is profoundly influenced by the expertise and innovative strategies of leading contract research organizations (CROs). Each organization highlighted—from bioaccess® to WuXi AppTec—exemplifies a steadfast commitment to enhancing the efficiency and effectiveness of clinical trials. By harnessing regional advantages, advanced technologies, and comprehensive service offerings, these CROs not only expedite the research process but also guarantee high-quality outcomes for biopharmaceutical companies.
Key insights from the article reveal that organizations like bioaccess® and ICON plc excel in:
While PPD and Parexel concentrate on:
Additionally, the focus on technology-driven strategies by Clinipace signifies a critical shift towards digital integration in clinical trials, highlighting the necessity of innovation in this competitive arena. The collaborative efforts of these organizations establish them as essential partners in navigating the complexities of clinical research.
As the demand for efficient and effective clinical trials escalates, the role of these premier contract research organizations becomes increasingly crucial. Stakeholders in the biopharmaceutical sector are urged to explore partnerships with these CROs to leverage their expertise, ultimately propelling advancements in healthcare and accelerating the delivery of innovative therapies to the market. Investing in these collaborations not only enhances research capabilities but also fosters a more agile and responsive clinical research environment, ensuring that groundbreaking medical solutions reach patients promptly.
What is bioaccess and what makes it unique in clinical research?
Bioaccess is a clinical research organization that leverages the regulatory efficiency of Latin America and its diverse patient demographics. It secures ethical approvals in 4-6 weeks and achieves patient enrollment 50% faster than traditional markets, resulting in significant cost savings.
How does bioaccess contribute to cost savings in clinical research?
Bioaccess estimates that conducting experiments in Latin America can reduce costs by over 30% compared to North America or Western Europe, alongside R&D tax incentives in Colombia, including a 100% tax deduction and various financial grants.
What is the quality of healthcare in Colombia regarding clinical trials?
Colombia's healthcare system is ranked #22 by the World Health Organization, with hospitals recognized as some of the best in Latin America. Additionally, hospitals must complete a stringent ICH/GCP certification process to conduct medical studies, ensuring high-quality standards.
What experience does bioaccess have in clinical trials?
Bioaccess has over 15 years of experience in early-stage trials, providing customized, high-quality services that help Medtech, Biopharma, and Radiopharma innovators accelerate their breakthroughs.
What are the benefits of partnering with bioaccess?
Collaborators with bioaccess report a 50% reduction in recruitment time and 95% retention rates in research studies, highlighting the effectiveness of their approach.
What services does IQVIA provide in clinical research?
IQVIA offers advanced analytics, regulatory knowledge, and extensive clinical research services throughout the medical device research lifecycle, focusing on site feasibility, investigator selection, and regulatory compliance.
How does IQVIA leverage Colombia's advantages for clinical trials?
IQVIA capitalizes on Colombia's cost reductions exceeding 30% compared to North America and Western Europe, along with a rapid IRB/EC and MoH review process of 90-120 days and a high-quality healthcare system.
What is the 'Patient Velocity' feature offered by IQVIA?
The 'Patient Velocity' feature enables 50% quicker enrollment of treatment-naive groups, leading to substantial cost savings of $25K per patient with FDA-ready data.
What services does ICON plc offer for clinical trial management?
ICON plc provides a comprehensive suite of study management services, including study design, regulatory submission, and management of complex trials across various therapeutic areas, with a strong focus on efficiency.
How quickly can ICON secure ethical approvals for clinical trials?
ICON can secure ethical approvals in just 4-6 weeks, significantly accelerating the clinical development process.
What is the scale of ICON plc and its financial performance?
ICON has approximately 41,900 employees across 106 locations in 55 countries. In 2024, they reported a full-year adjusted EBITDA of $1,735.8 million, representing 21.0% of revenue, with a net book-to-bill ratio of 1.20.
How does bioaccess compare to ICON in terms of patient enrollment speed?
Bioaccess can enroll treatment-naive cardiology or neurology groups 50% faster than Western locations, establishing a competitive advantage in the research landscape.