

This article presents a comprehensive step-by-step guide for implementing UDI barcodes in Mexico, with a strong focus on regulatory compliance and operational efficiency for healthcare providers. It underscores the critical importance of understanding registration requirements, labeling standards, and the effective integration of IT systems. Additionally, it addresses common challenges such as barcode scanning issues and staff resistance, thereby facilitating a successful UDI implementation process.
The implementation of Unique Device Identification (UDI) in Mexico marks a pivotal shift in the healthcare landscape, significantly enhancing patient safety and operational efficiency through improved traceability and compliance.
As medical providers navigate the complexities of UDI barcode systems, they stand to gain substantial benefits, including:
However, the journey toward successful implementation is fraught with challenges, ranging from:
How can providers effectively adopt UDI while overcoming these obstacles and ensuring compliance with the evolving regulations?
Unique Product Identification (UDI) serves as a crucial framework that assigns a distinct identifier to medical products, enabling their recognition throughout the supply chain. This system significantly enhances patient safety by improving traceability, streamlining recalls, and minimizing medical errors. The UDI framework consists of two elements: the Device Identifier (DI), which indicates the version or model of an item, and the Production Identifier (PI), which contains essential details such as the lot or serial number. For manufacturers, grasping the nuances of UDI is essential not only for regulatory compliance but also for optimizing operational efficiency.
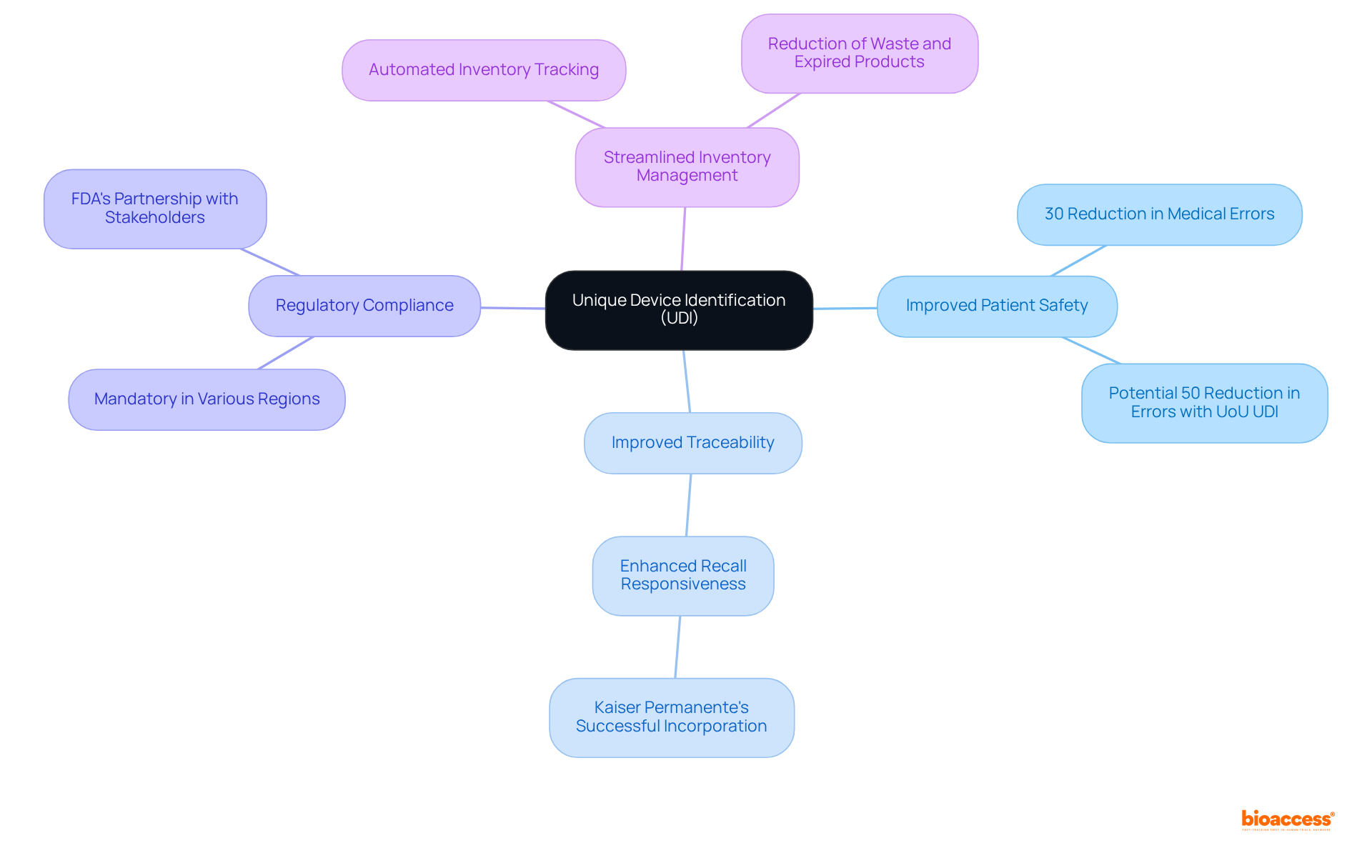
Key Benefits of UDI:
The UDI framework is not merely a regulatory requirement; it is a vital tool for improving patient safety and operational efficiency in healthcare. Furthermore, the role of surgeons in promoting UDI implementation is crucial, as they are essential stakeholders in ensuring the framework's success within healthcare institutions. Despite its benefits, challenges such as high costs and the need for system upgrades can hinder widespread adoption. As mentioned by specialists, cooperation among healthcare providers, manufacturers, and oversight organizations is crucial for surmounting these obstacles and completely achieving the benefits of UDI.
In Mexico, the regulatory framework for Unique Device Identification (UDI) is overseen by COFEPRIS (Federal Commission for Protection against Sanitary Risks). To effectively navigate this landscape, manufacturers must grasp several critical aspects.
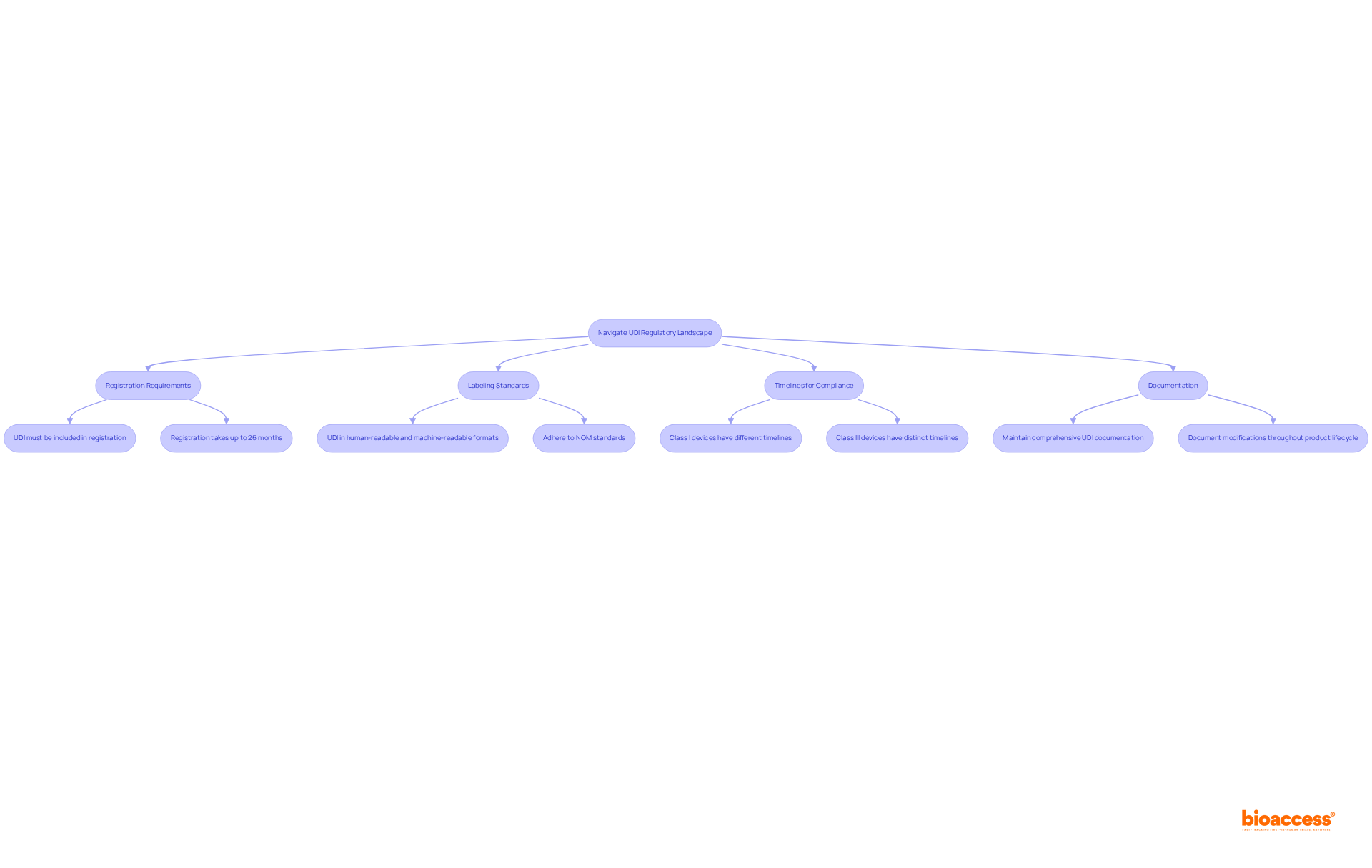
Registration Requirements: All medical products must be registered with COFEPRIS, and the UDI must be included in the registration documentation. This is essential for compliance and market access. The total timeline for registration can be as long as 26 months in the worst-case scenario, emphasizing the importance of understanding compliance timelines.
Labeling Standards: The UDI must be prominently displayed on the label of the equipment in both human-readable and machine-readable formats. Adherence to the latest NOM (Official Mexican Standards) regarding labeling is crucial to ensure that products meet regulatory expectations.
Timelines for Compliance: It is essential to be aware of the deadlines for UDI implementation, which vary by class of equipment. For example, Class I devices may have distinct timelines compared to Class III devices, necessitating careful planning to meet these requirements. Recent updates to COFEPRIS regulations in 2025 have refined classification criteria, providing a more current context for manufacturers.
Documentation: Maintaining comprehensive documentation of all UDI-related processes is imperative. This includes the creation of UDIs, labeling practices, and any modifications made throughout the product lifecycle. Thorough documentation not only aids in compliance but also mitigates the risk of penalties. Manufacturers should also be aware that government fees associated with the registration process range from $1,000 to $2,000 USD.
By comprehending these governing requirements, manufacturers can ensure compliance with COFEPRIS and navigate the complexities of the UDI landscape effectively. As noted by bioaccess®, "Accurate classification is crucial for successfully navigating the regulatory landscape, as it determines the regulatory requirements and processes that manufacturers must follow for their products." Furthermore, it's crucial to acknowledge that imports comprise almost 90% of medical equipment sold in Mexico, emphasizing the competitive environment and the dependence on imports.
The udi barcode implementation mexico provider plays a crucial role in implementing UDI barcode systems, which involves several critical steps essential for compliance and operational efficiency.
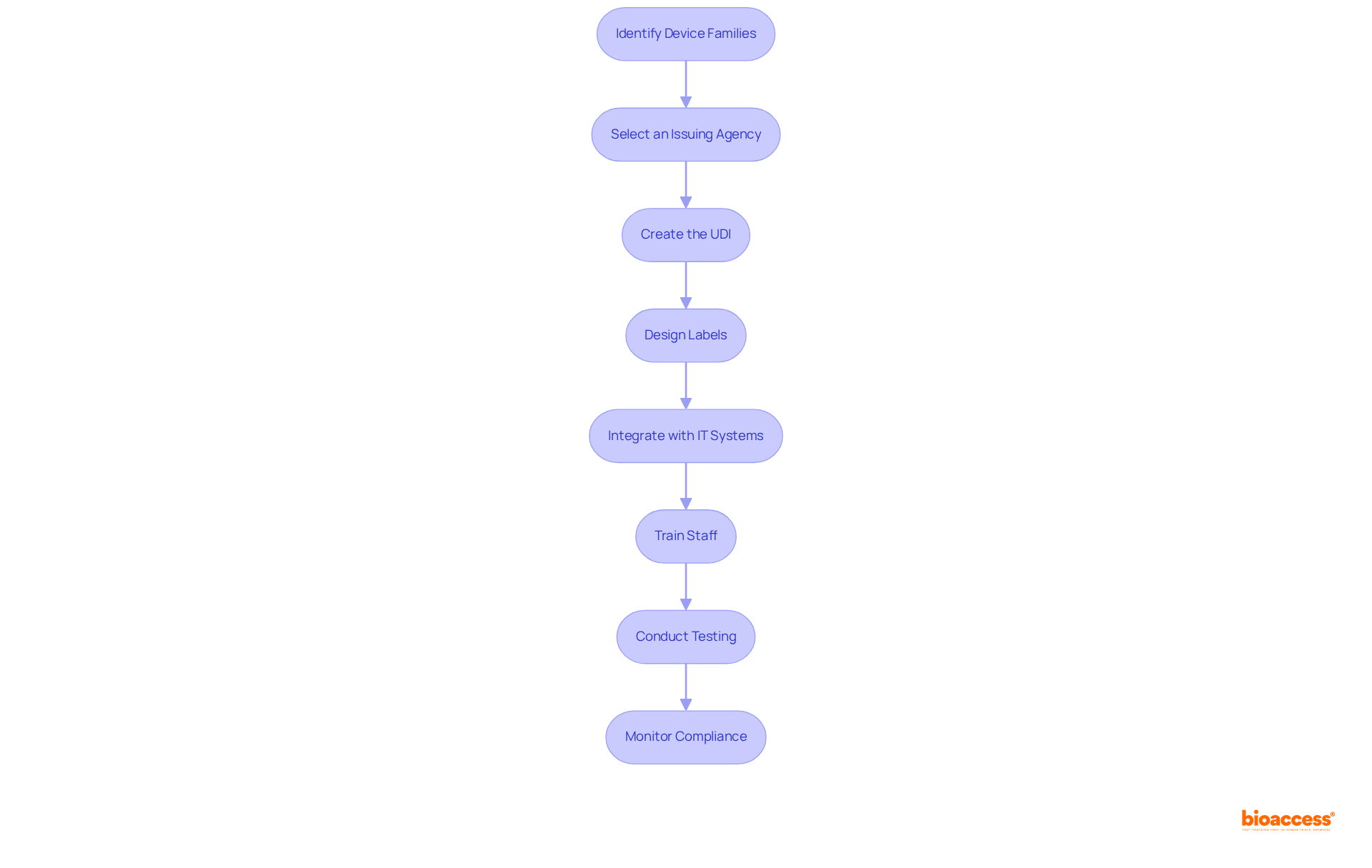
Identify Device Families: Determine which devices require UDI and categorize them into families based on their intended use and risk classification. This structured approach ensures compliance is effectively managed.
Select an Issuing Agency: Choose an FDA-accredited issuing agency to obtain your Device Identifier (DI). This step is crucial for ensuring that your UDI barcode implementation Mexico provider is recognized and meets compliance standards.
Create the UDI: Generate the UDI following the guidelines of the selected agency. It is vital that both the Device Identifier (DI) and the Production Identifier (PI) are formatted correctly, as this is essential for traceability.
Design Labels: Create labels that display the UDI in both human-readable and machine-readable formats, such as barcodes. Adhering to COFEPRIS labeling standards is mandatory for the udi barcode implementation mexico provider to avoid regulatory issues.
Integrate with IT Systems: Update your inventory management and electronic health record systems to accommodate UDI data. This integration with the udi barcode implementation mexico provider will enhance supply chain efficiency and improve patient safety by ensuring precise tracking of equipment.
Train Staff: Conduct comprehensive training sessions for staff on UDI requirements, barcode scanning, and data entry processes. Effective training fosters a culture of adherence and minimizes compliance risks, especially for a udi barcode implementation mexico provider. Moreover, develop a comprehensive data management plan and implement effective data quality control processes to ensure successful UDI data submission and compliance.
Conduct Testing: Test the barcode scanning process to ensure that all UDIs are readable and accurately linked to their corresponding equipment. This step is vital for maintaining data integrity and compliance with the udi barcode implementation Mexico provider.
Monitor Compliance: Regularly review and update UDI processes to ensure ongoing compliance with regulatory changes. Continuous monitoring is essential for the udi barcode implementation mexico provider to adapt to evolving standards and maintain operational efficiency. Be aware that missed compliance deadlines can result in penalties or delays in product approvals, underscoring the importance of adhering to UDI requirements.
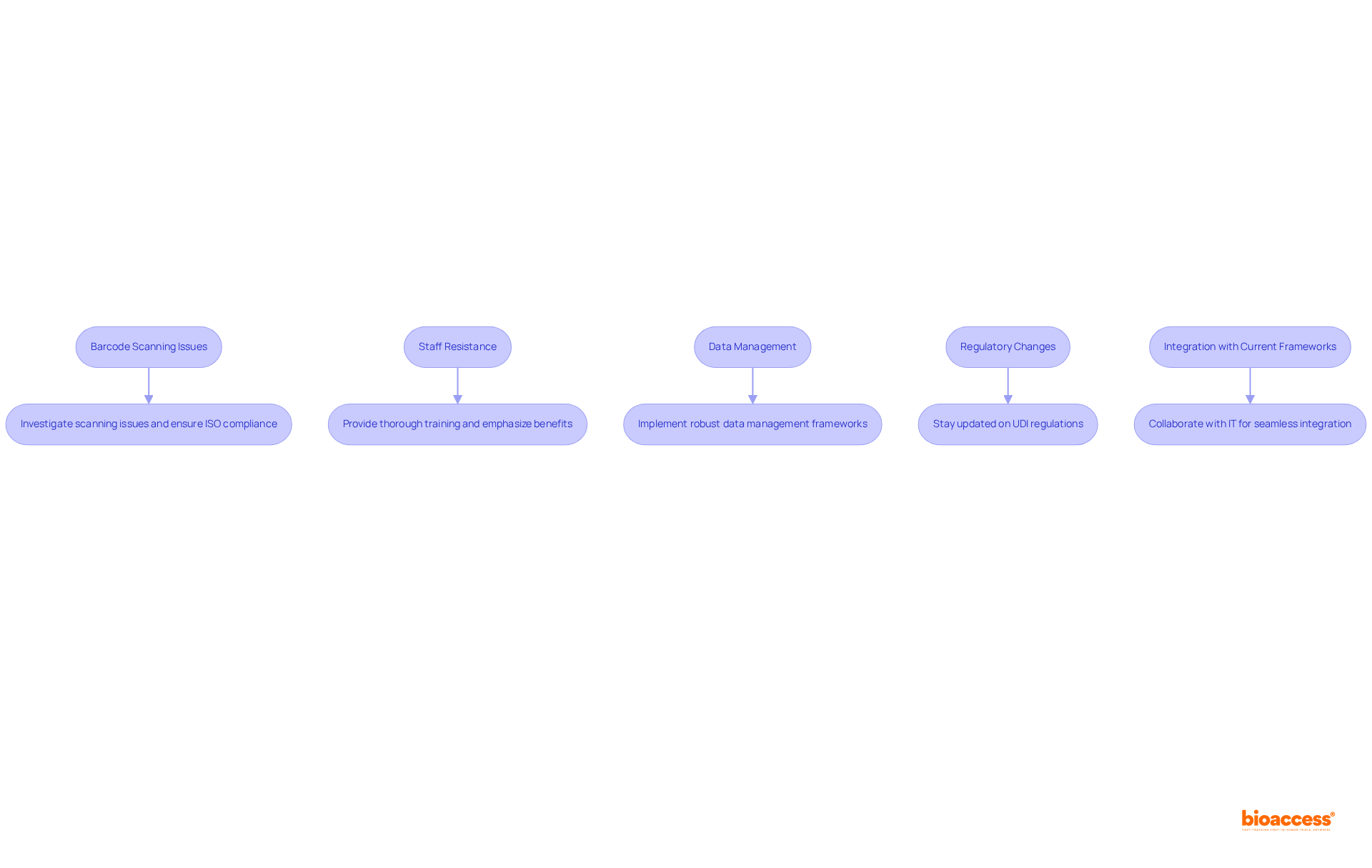
Despite meticulous planning, challenges may emerge during the UDI barcode implementation Mexico provider. Below are some prevalent issues along with their resolutions:
Barcode Scanning Issues: If barcodes fail to scan accurately, investigate common issues such as low contrast, incorrect symbology, or label damage. It is crucial to ensure that the barcode adheres to ISO standards. A recent assessment revealed that over 12,000 barcodes were scanned, underscoring the significance of proper scanning practices. As Beth Wells, a UDI and medical device expert at GS1 US, emphasizes, "It’s important to assess your hardware. Be aware of the different barcodes and have your scanner programmed for these."
Staff Resistance: Employees may exhibit resistance to the changes associated with UDI implementation. This challenge can be mitigated by offering thorough training and emphasizing the benefits of the UDI barcode implementation Mexico provider for patient safety and operational efficiency.
Data Management: The maintenance of accurate UDI data presents its own set of challenges. Handling large volumes of UDI data can be daunting and susceptible to inaccuracies. It is essential for the UDI barcode implementation Mexico provider to implement robust data management frameworks to consistently monitor and refresh UDI information.
Regulatory Changes: To remain compliant, it is vital to stay updated on changes in UDI regulations by regularly consulting COFEPRIS updates and industry news related to the UDI barcode implementation Mexico provider. Adapting processes in accordance with these changes is crucial.
Integration with Current Frameworks: Ensure that your IT infrastructure is equipped to handle UDI data. Integration challenges often arise from the necessity of multiple data pool communications for UDI compliance. Collaborate with IT professionals to seamlessly integrate UDI into your existing inventory and tracking systems.
The implementation of Unique Device Identification (UDI) barcodes in Mexico is not merely a regulatory obligation; it represents a transformative step towards enhancing patient safety and operational efficiency within the healthcare sector. Understanding the significance of UDI and navigating the complexities of the regulatory landscape are essential for manufacturers aiming to optimize their operations while ensuring compliance.
Key insights from the article underscore the multifaceted benefits of UDI, including:
The step-by-step guide offers a clear framework for successful UDI barcode implementation, addressing common challenges such as:
By fostering collaboration among healthcare providers, manufacturers, and regulatory bodies, the potential of UDI can be fully realized, leading to safer healthcare practices.
Ultimately, embracing UDI is a critical investment in the future of healthcare in Mexico. It not only enhances the quality of care provided to patients but also positions manufacturers to thrive in a competitive market. As the landscape evolves, staying informed about regulatory updates and best practices will be vital for maintaining compliance and maximizing the benefits of UDI implementation. Taking proactive steps now can ensure that healthcare providers are well-equipped to meet the challenges ahead and contribute to a safer, more efficient healthcare environment.
What is Unique Device Identification (UDI)?
Unique Device Identification (UDI) is a framework that assigns a distinct identifier to medical products, enhancing their recognition throughout the supply chain.
How does UDI improve patient safety?
UDI improves patient safety by enabling precise identification of devices used in patient care, which helps mitigate the risk of medical errors. For example, Mercy Health's implementation of UDI led to a 30% reduction in medical errors.
What are the two main components of the UDI framework?
The UDI framework consists of two components: the Device Identifier (DI), which indicates the version or model of an item, and the Production Identifier (PI), which includes essential details like the lot or serial number.
How does UDI enhance traceability in medical products?
UDI allows for thorough monitoring of medical products throughout their lifecycle, which is vital for effective recalls. Hospitals using UDI have reported improved responsiveness to device recalls.
Why is regulatory compliance important for UDI?
Adhering to UDI regulations is mandatory in various regions, ensuring that manufacturers meet safety and quality standards. The FDA collaborates with healthcare stakeholders to encourage the adoption of UDI.
What are the benefits of UDI for inventory management?
UDI automates inventory tracking, reducing manual errors and improving supply chain management. Hospitals that have implemented UDI technology have seen significant enhancements in inventory management practices.
What role do surgeons play in UDI implementation?
Surgeons are essential stakeholders in promoting UDI implementation within healthcare institutions, contributing to the framework's success.
What challenges exist in the widespread adoption of UDI?
Challenges to UDI adoption include high costs and the need for system upgrades. Cooperation among healthcare providers, manufacturers, and oversight organizations is crucial to overcoming these obstacles.