

The article underscores the critical importance of Clinical Data Management Systems (CDMS) in clinical research, highlighting their essential role in ensuring data accuracy, regulatory compliance, and operational efficiency. It outlines key functionalities such as:
These elements work in concert to enhance decision-making and expedite the research process. Ultimately, this leads to improved patient outcomes and reduced costs, reinforcing the necessity of CDMS in the evolving landscape of clinical research.
The landscape of clinical research is rapidly evolving, underscoring the increasing demand for precise and reliable data management. A Clinical Data Management System (CDMS) emerges as a pivotal tool in this transformation, streamlining the collection and analysis of health information while ensuring compliance with stringent regulatory standards. As researchers endeavor to enhance trial outcomes and accelerate patient enrollment, one must consider:
A Clinical Information Management System serves as an advanced software solution meticulously designed to manage the information generated during trials and studies. This system acts as a centralized hub for the collection, validation, and analysis of health information, ensuring that the details are not only accurate but also secure and compliant with stringent regulatory standards.
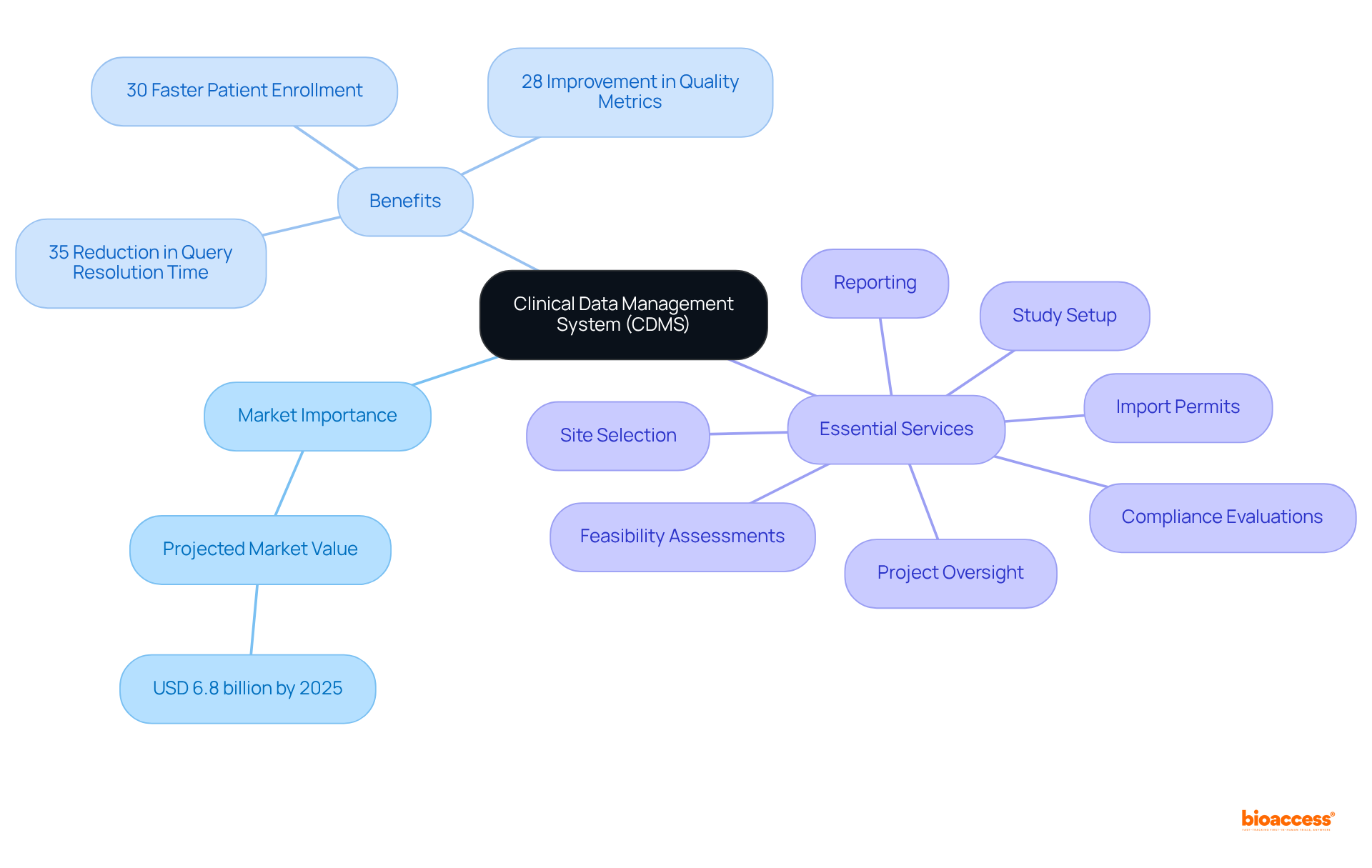
By 2025, the market for data management systems is projected to reach approximately USD 6.8 billion, highlighting its growing importance in the research landscape. This system plays a pivotal role in optimizing the research process, enabling effective information management throughout the study lifecycle.
Effective applications of content management systems, particularly those utilizing AI-driven information management solutions, have demonstrated significant benefits, including:
Furthermore, research studies that incorporate integrated, automated data management processes complete patient enrollment 30% more rapidly. Experts assert that the integration of clinical data management systems, or cdms meaning, enhances decision-making capabilities, ultimately leading to faster patient enrollment and more reliable research outcomes.
Additionally, comprehensive research study management services—including:
are essential for the success of research investigations. These services not only ensure compliance with regulatory standards but also encompass critical processes such as the review and feedback on study documents and the import permit process. This contributes to local economies through job creation, economic growth, healthcare improvement, and international collaboration.
As the complexity of medical studies continues to escalate, the role of management systems for clinical information becomes increasingly vital, establishing it as an indispensable resource for researchers and organizations aiming to refine their management strategies.
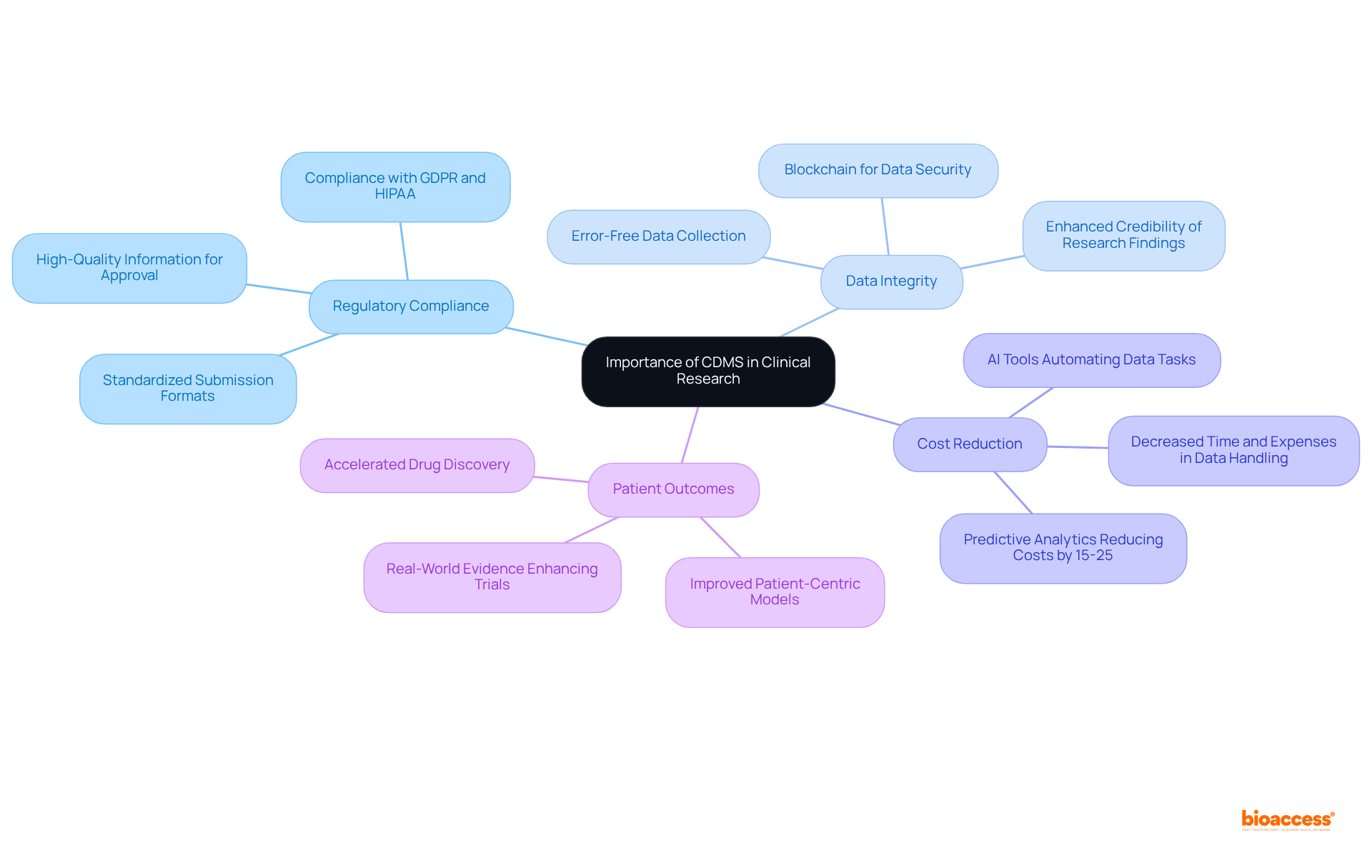
The CDMS meaning highlights the paramount importance of a Clinical Data Management System in clinical research. This system ensures that information gathered during trials is not only precise and comprehensive but also easily accessible for analysis. Such capability is vital for achieving regulatory compliance, as governing bodies require high-quality information for the approval of new drugs and medical devices.
A well-executed content management system can lead to significant decreases in both time and expenses related to information handling, allowing researchers to concentrate on their primary tasks and accelerate the creation of groundbreaking therapies. By ensuring data integrity, the system enhances the credibility of research findings, ultimately contributing to improved patient outcomes.
Moreover, the CDMS meaning is crucial as the incorporation of predictive analytics within clinical data management systems can reduce study expenses by 15-25%, making it an attractive option for sponsors and contract research organizations (CROs) seeking to lower costs. As the clinical trial environment evolves, implementing clinical data management systems becomes increasingly essential for organizations aiming to uphold compliance with strict regulatory standards while improving operational efficiency.
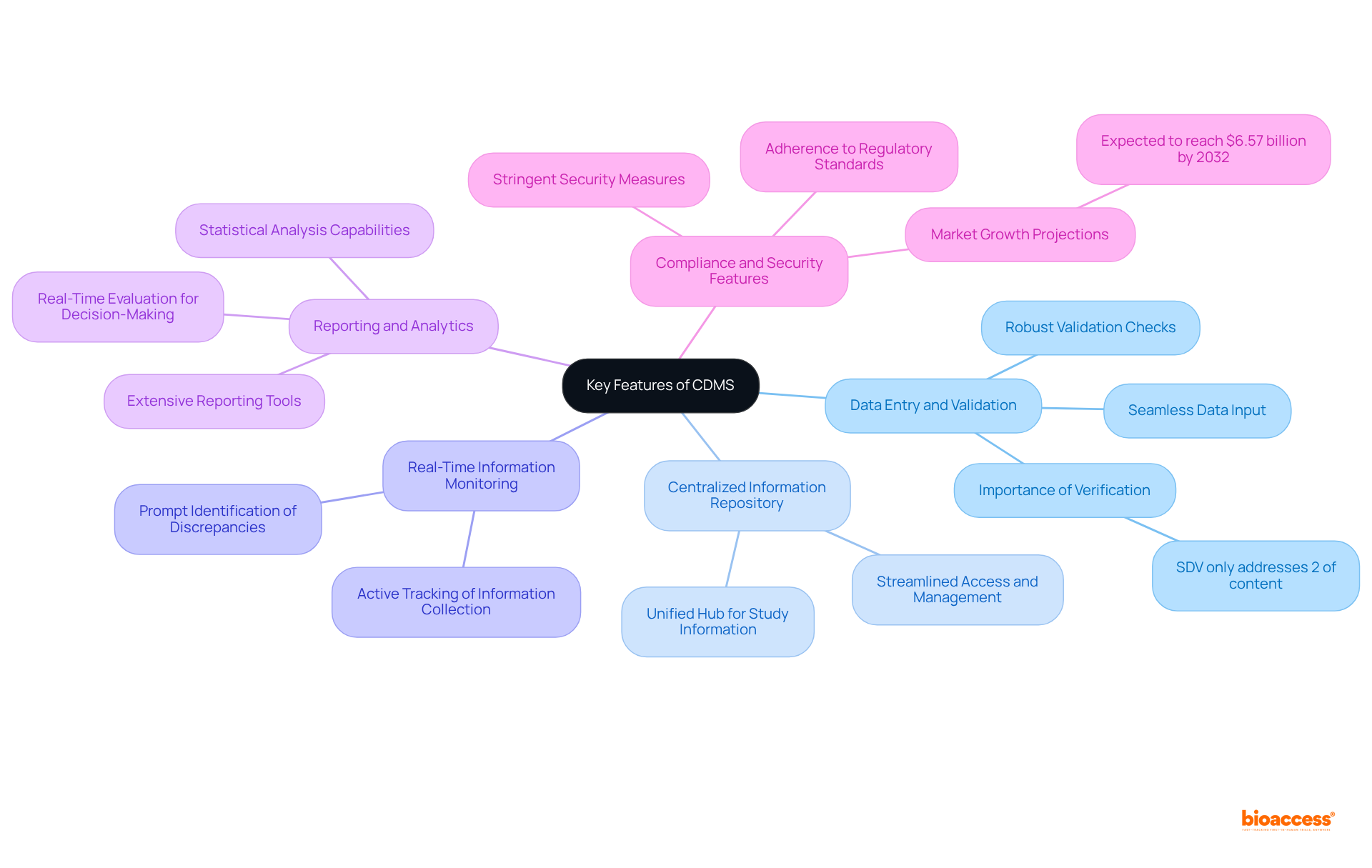
Key features and functionalities of a Clinical Data Management System (CDMS) encompass several critical components:
Data Entry and Validation: The CDMS facilitates seamless data input, integrating robust validation checks to ensure accuracy and consistency. This aspect is vital, as studies reveal that source information verification (SDV) addresses only about 2% of the content, underscoring the necessity for rigorous validation procedures.
Centralized Information Repository: Acting as a unified hub for all study information, the system streamlines access and management, empowering researchers to effectively handle substantial amounts of data.
Real-Time Information Monitoring: Many clinical information management systems are equipped with real-time monitoring capabilities, allowing researchers to actively track information collection and promptly identify discrepancies. This functionality is crucial for maintaining information integrity and enhancing procedural efficiency.
Reporting and Analytics: The system provides extensive tools for generating reports and conducting statistical analyses, essential for interpreting study outcomes. The ability to evaluate information in real-time supports informed decision-making throughout the research process.
Compliance and Security Features: With stringent security measures in place, the system safeguards sensitive patient information while ensuring adherence to regulatory standards. This is increasingly critical as the market for content management systems is projected to expand significantly, nearing $6.57 billion by 2032, driven by the demand for information accuracy and compliance in research studies. Furthermore, advancements such as generative AI are reducing process costs by up to 50%, thereby enhancing the efficiency of information management systems.
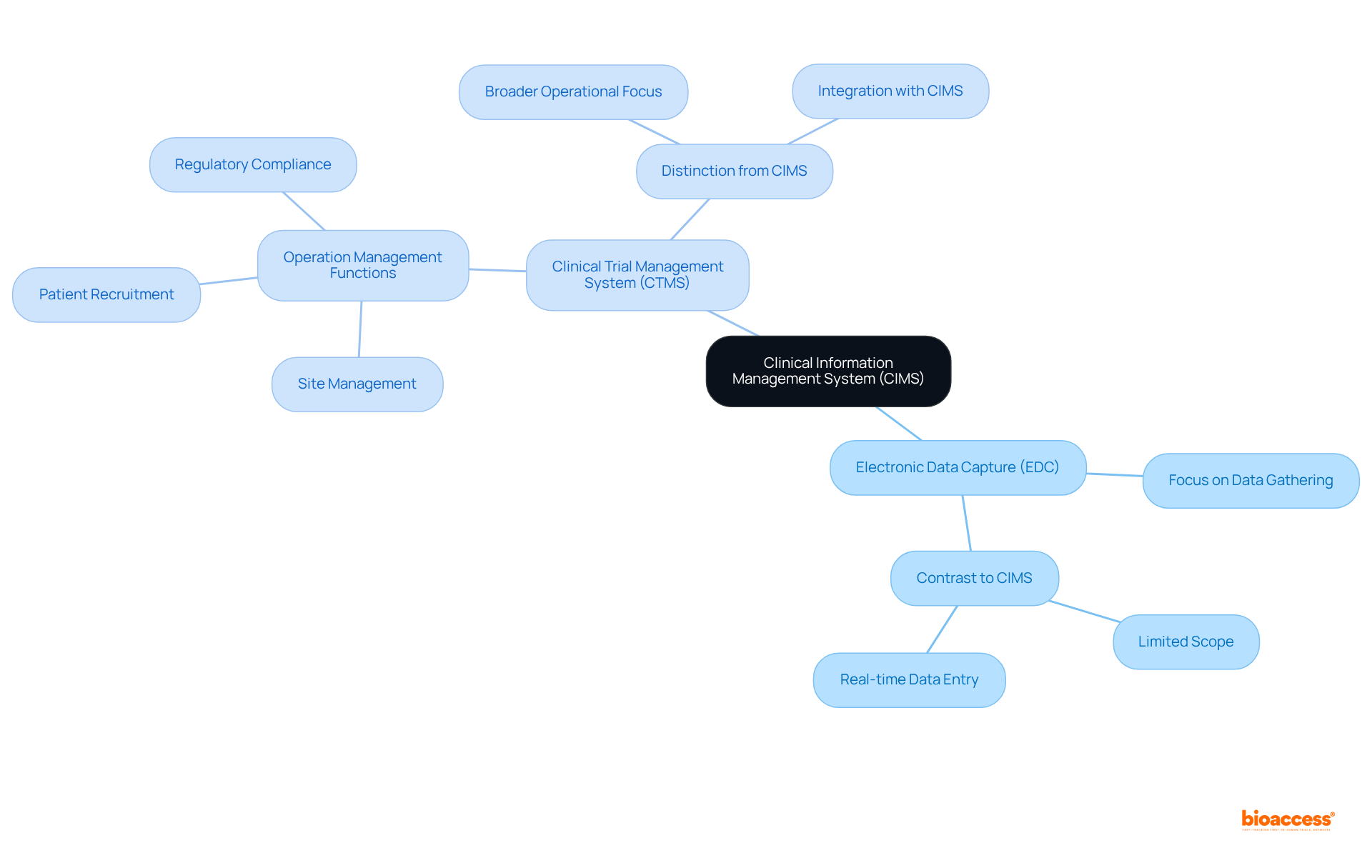
A Clinical Information Management System (CIMS) is pivotal in overseeing and ensuring the quality of trial information. However, it is essential to differentiate it from similar systems.
Electronic Data Capture (EDC): EDC systems primarily focus on the front-end information gathering process, enabling researchers to input data directly from research sites. In contrast, CIMS encompasses a broader range of functionalities, including validation, cleaning, and analysis, ensuring that the information gathered is both precise and trustworthy.
Clinical Trial Management System (CTMS): CTMS is designed to supervise the overall operations of research studies, including site management, patient recruitment, and regulatory compliance. While CTMS may incorporate some information management capabilities, CIMS is specifically tailored to meet the stringent requirements of integrity and quality assurance in research studies.
Understanding these distinctions is crucial for selecting the appropriate tools for efficient trial management. The integration of CIMS with CTMS is becoming increasingly common, as organizations strive to streamline operations and enhance data accuracy. In fact, the clinical data management systems market is projected to experience significant growth, with a compound annual growth rate (CAGR) of 13.6% from 2024 to 2031, reflecting the rising demand for effective data management solutions in clinical research.
The significance of a Clinical Data Management System (CDMS) in clinical research is paramount. This advanced software solution acts as a cornerstone for managing the extensive data generated during trials, ensuring that the information remains accurate, secure, and compliant with regulatory standards. As the demand for effective data management solutions escalates, the CDMS stands out as an essential tool for researchers aiming to enhance the quality and efficiency of clinical studies.
Throughout this article, we have highlighted key insights into the functionalities and benefits of CDMS. From facilitating seamless data entry and validation to providing real-time monitoring and comprehensive reporting capabilities, a CDMS not only streamlines information management but also significantly enhances decision-making and operational efficiency. The integration of predictive analytics further reduces costs and accelerates the research process, ultimately leading to faster patient enrollment and more reliable outcomes.
Given these advantages, it is crucial for organizations involved in clinical research to recognize the transformative potential of CDMS. By adopting this technology, researchers can ensure compliance with stringent regulatory requirements while bolstering the integrity of their studies. As the landscape of clinical trials evolves, embracing a robust CDMS will be pivotal in driving innovation and improving patient care, making it an essential investment for the future of healthcare research.
What is a Clinical Data Management System (CDMS)?
A Clinical Data Management System (CDMS) is an advanced software solution designed to manage information generated during clinical trials and studies. It serves as a centralized hub for the collection, validation, and analysis of health information, ensuring accuracy, security, and compliance with regulatory standards.
What is the projected market value for data management systems by 2025?
The market for data management systems is projected to reach approximately USD 6.8 billion by 2025, indicating its growing importance in the research landscape.
What benefits do AI-driven content management systems provide?
AI-driven content management systems have demonstrated significant benefits, including a 35% reduction in query resolution time and a 28% improvement in quality metrics.
How do integrated and automated data management processes affect patient enrollment?
Research studies that utilize integrated and automated data management processes complete patient enrollment 30% more rapidly, enhancing overall efficiency.
What are some essential services included in comprehensive research study management?
Essential services include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting.
How do these management services contribute to research investigations?
These services ensure compliance with regulatory standards, facilitate the review and feedback on study documents, and support the import permit process, contributing to local economies through job creation, economic growth, healthcare improvement, and international collaboration.
Why is the role of clinical information management systems becoming increasingly vital?
As the complexity of medical studies escalates, clinical information management systems are essential for researchers and organizations to refine their management strategies and enhance decision-making capabilities.