


The clinical evidence requirements for medical devices in Mexico, as regulated by COFEPRIS, necessitate comprehensive research data that demonstrates both safety and effectiveness. These requirements vary based on the risk classification of the device, ensuring that each product meets the necessary standards.
By detailing these requirements, the article illustrates how they serve to approve only safe and effective healthcare products, thereby safeguarding patient well-being. This not only enhances confidence in healthcare technologies but also underscores the critical role of rigorous clinical evidence in the Medtech landscape.
Navigating the regulatory landscape for medical devices in Mexico presents a complex challenge for manufacturers and stakeholders alike. The Federal Commission for Protection against Sanitary Risk (COFEPRIS) oversees stringent clinical evidence requirements, making it crucial to understand these regulations to ensure product safety and efficacy. This article delves into the essential components of clinical evidence, the risk classification of devices, and the anticipated changes in 2025. It also highlights the obstacles that producers face in meeting these requirements.
How can manufacturers effectively navigate this intricate framework and ensure compliance in an increasingly competitive market?
In Mexico, the clinical evidence requirements for medical devices are established and regulated by COFEPRIS (Federal Commission for Protection against Sanitary Risk). Producers are mandated to provide comprehensive research data that fulfills the clinical evidence requirements for Mexico devices, ensuring the safety and effectiveness of their products. This data must be derived from rigorously designed clinical trials or studies that fulfill the clinical evidence requirements for Mexico devices and should directly support the performance claims made by the manufacturer.
Furthermore, the clinical evidence requirements for devices in Mexico must align with the device's risk classification, which ranges from low-risk Class I to high-risk Class IV. Notably, approximately 70% of healthcare instruments submitted for approval in Mexico fall under Class I, typically requiring less extensive documentation compared to higher-risk categories.
The evaluation period for health equipment registration in 2025 is anticipated to range from 3 to 8 months, with the option of utilizing an Authorized Third Party to expedite registration to as little as 1 to 3 months. Additionally, all documentation submitted to COFEPRIS, including foreign clinical study data, must be translated into Spanish.
The primary aim of the clinical evidence requirements for Mexico devices is to ensure that only safe and effective healthcare products are approved for use within the Mexican health system, thereby protecting patient well-being and enhancing confidence in healthcare technologies.
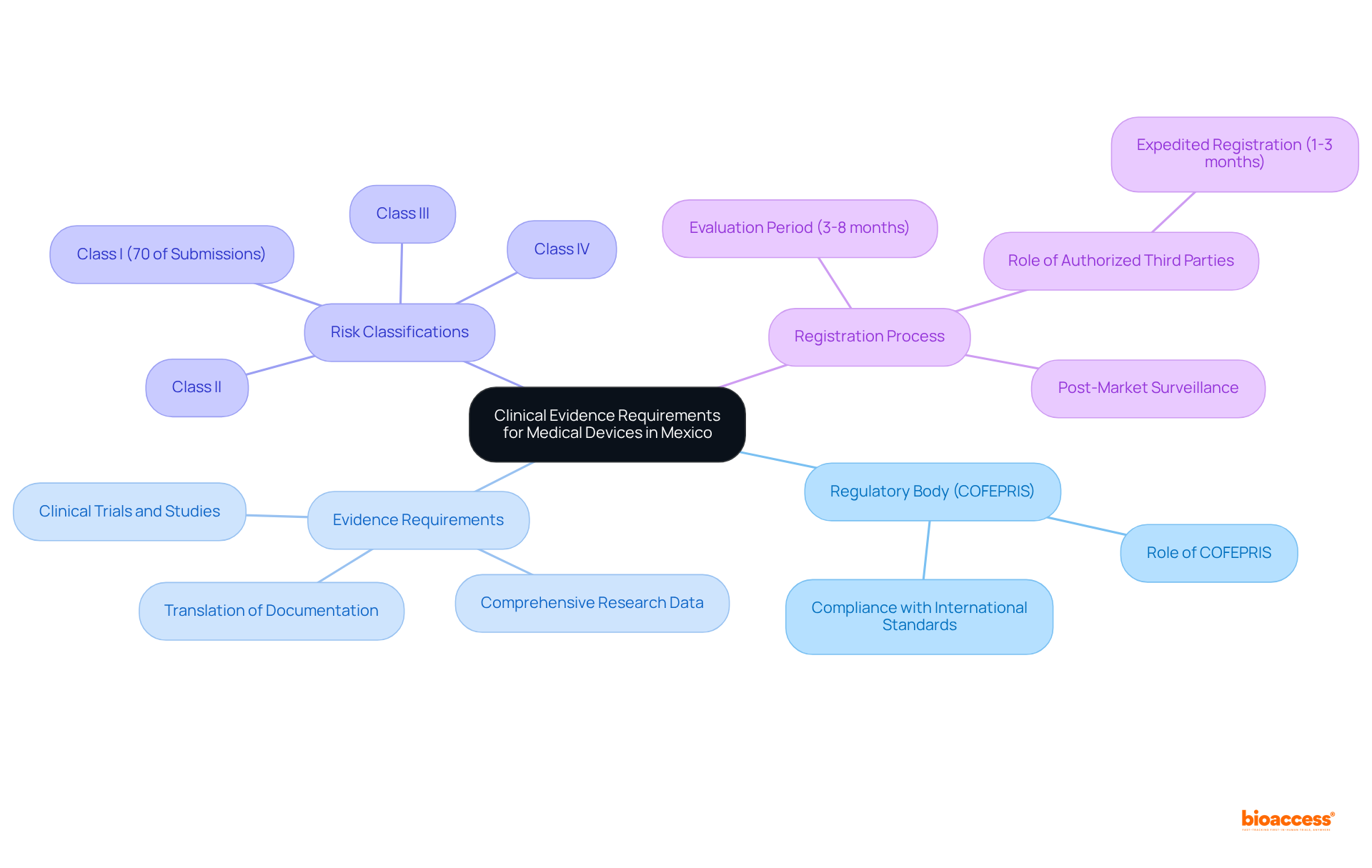
The regulatory framework governing medical equipment in Mexico is primarily established by the General Health Law and enforced by COFEPRIS, which is crucial in ensuring the safety and efficacy of medical products. Medical equipment is classified into three risk levels:
This classification significantly influences the type and extent of medical evidence required for each category. For example, higher-risk instruments necessitate extensive clinical data to demonstrate safety and effectiveness, whereas lower-risk instruments may have more streamlined requirements.
Moreover, Mexico has formed equivalence agreements with various countries, streamlining the registration process. This arrangement allows for expedited approvals of equipment that has received authorization in regions with stringent regulatory standards. Such initiatives are designed to protect public health while fostering innovation within the healthcare equipment sector, ultimately enhancing access to advanced health technologies for the Mexican population.
As we look to 2024, the healthcare device regulatory affairs market in Mexico is projected to reach a revenue of USD 120.7 million by 2030, reflecting the growing demand for advanced healthcare technologies. Notably, nearly 90% of healthcare devices sold in Mexico are imports, underscoring the importance of understanding local regulations for successful market entry. Beginning in 2025, compliance with Good Manufacturing Practices will be mandatory for low-risk medical products, making it essential for manufacturers to remain updated on evolving regulations. Furthermore, appointing a local registration holder is vital for managing the enrollment process and liaising with COFEPRIS, ensuring timely approvals and effective market entry in the country.
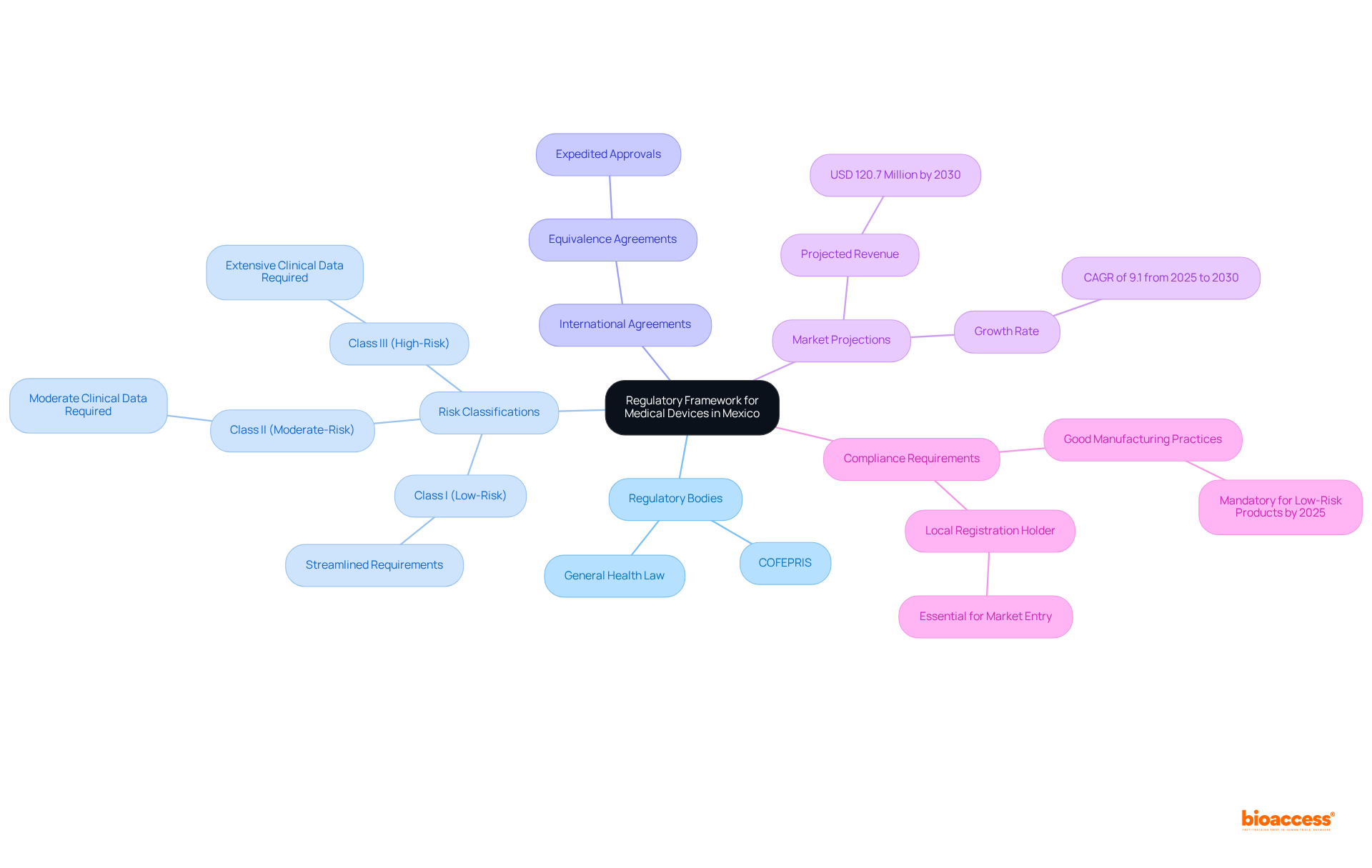
Key components of clinical evidence requirements in Mexico devices encompass several essential elements:
Medical data that demonstrates the safety and effectiveness of the equipment, sourced from trials, performance evaluations, or literature reviews. Clinical evaluation reports must incorporate a thorough analysis of available clinical evidence requirements in Mexico for devices regarding the safety and performance of the product. COFEPRIS requires manufacturers to submit published studies that comply with the clinical evidence requirements in Mexico for devices, ensuring they have adequate sample sizes to substantiate safety and efficacy.
Compliance with ISO 13485 standards or equivalent quality management systems ensures that manufacturing processes adhere to international safety and quality benchmarks. This compliance is crucial, as it can lead to increased market share and a positive brand reputation, while expediting the regulatory approval process and reducing the likelihood of product recalls by proactively addressing potential issues.
Comprehensive technical documentation outlines the product's intended use, design specifications, and labeling information, which is essential for regulatory approval.
A comprehensive risk assessment identifies potential dangers related to the equipment and outlines effective mitigation strategies. Together, these elements guarantee that the device meets the required safety and performance criteria before being sold in the country.
Additionally, ethical approvals are delivered in 4-6 weeks, highlighting the efficiency of the approval process in Mexico. Maintaining close contact with the research leader and the study group is also crucial for successful trials.
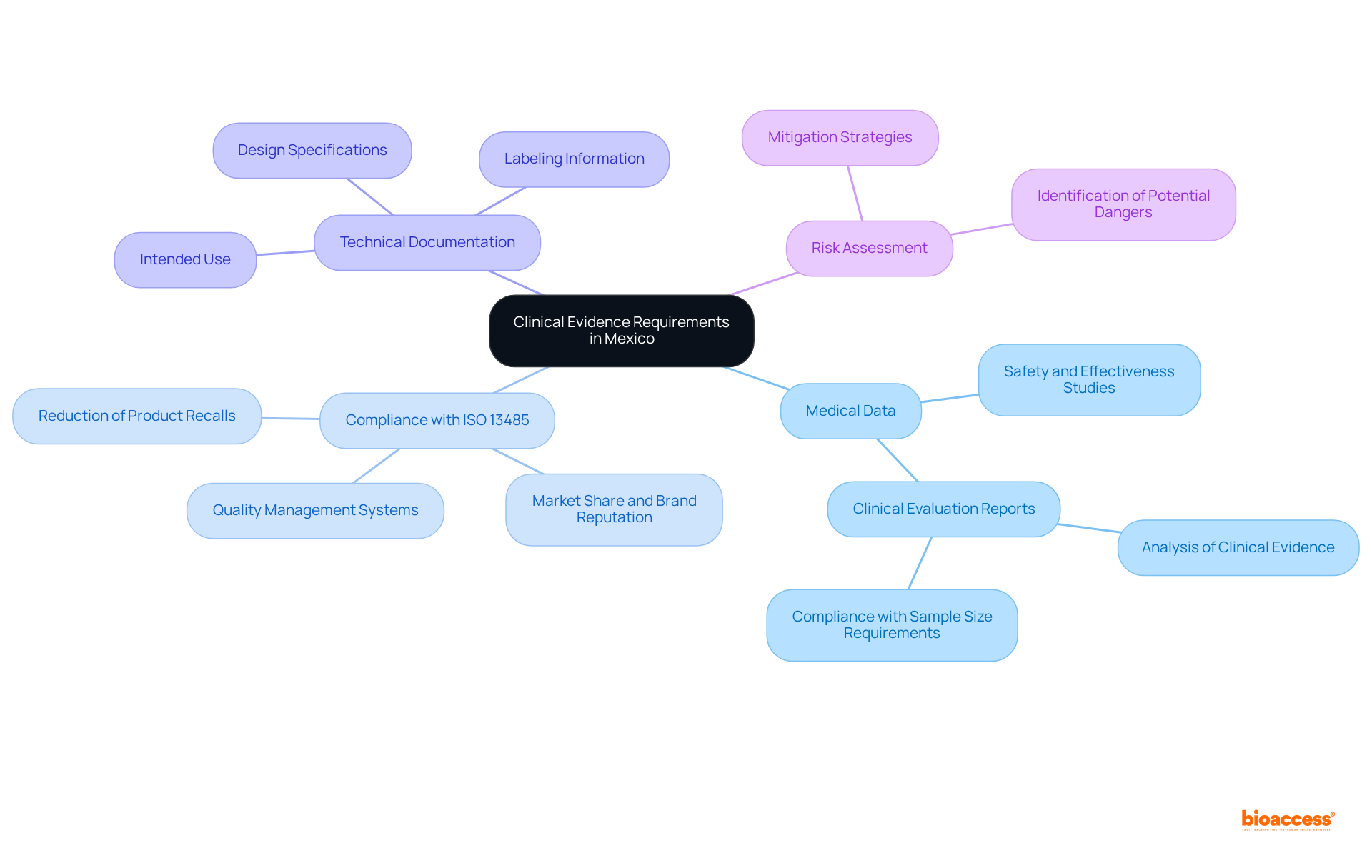
Producers face significant challenges in meeting evidence requirements in Mexico, primarily due to the complex regulatory landscape overseen by COFEPRIS. This intricacy can be overwhelming for those unfamiliar with the guidelines, often resulting in increased costs and extended approval timelines. The demand for extensive medical data complicates the process further, requiring considerable resources and time investment. Notably, the Mexico medical device research trials market generated USD 374.6 million in 2024 and is projected to reach USD 443.2 million by 2030, highlighting the growing necessity for effective evidence strategies.
Moreover, the recruitment of suitable participants for research trials presents another considerable hurdle, particularly in regions characterized by diverse populations. Cultural differences and socioeconomic factors can significantly impact participation rates, necessitating the implementation of culturally sensitive recruitment strategies. Community-centered approaches that engage local organizations have proven effective in fostering trust and encouraging participation, with 62.4% of Hispanic individuals expressing interest in home visits for research studies.
Language barriers further complicate the process, especially during informed consent, underscoring the need for bilingual staff to ensure clear communication. To navigate these challenges successfully, manufacturers are encouraged to collaborate with local experts and regulatory consultants who can offer invaluable guidance and support throughout the approval process. Implementing comprehensive information management systems can enhance the likelihood of regulatory approval by 23%, thereby streamlining compliance with COFEPRIS regulations and improving the chances of successful clinical trials in Mexico.
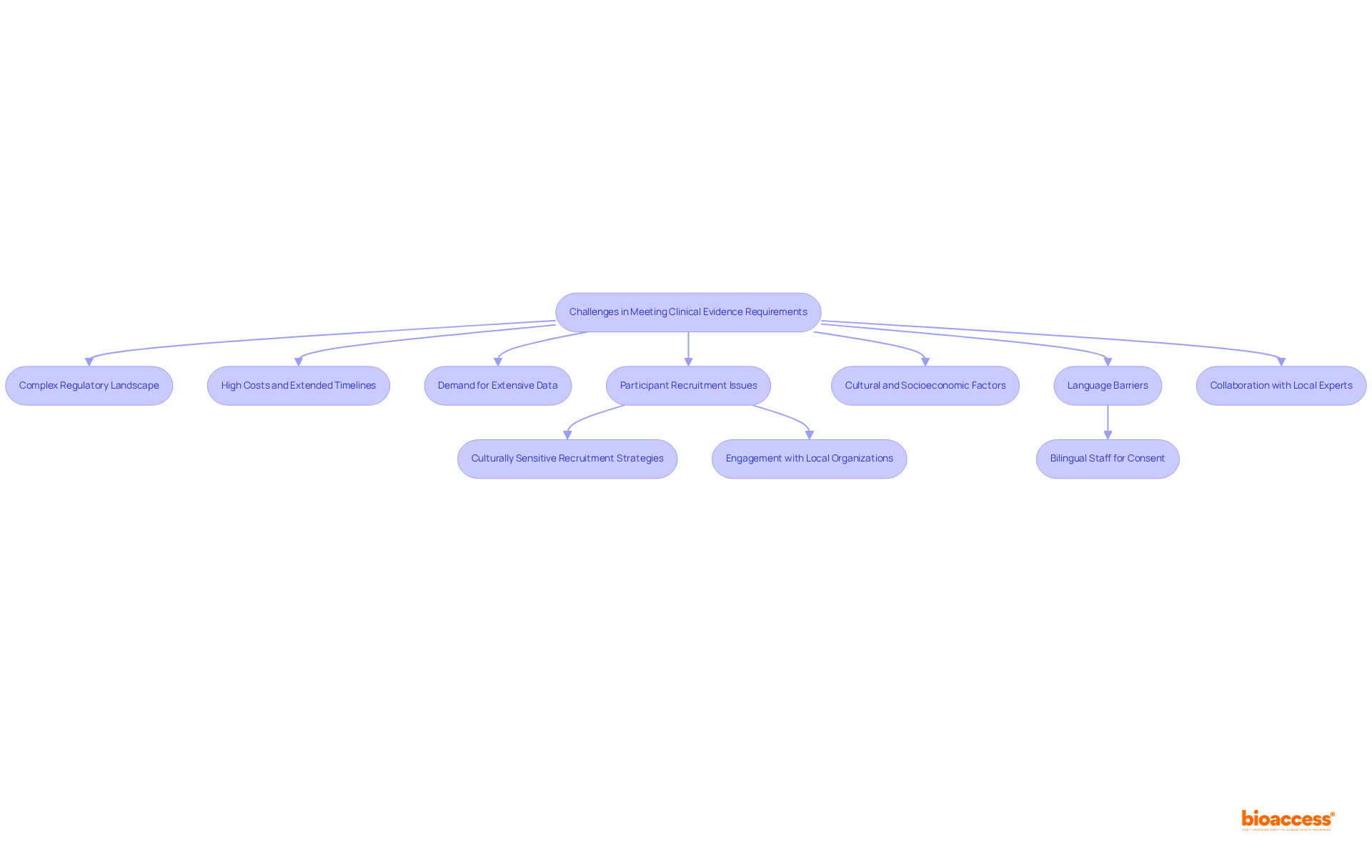
The clinical evidence requirements for medical devices in Mexico are pivotal in guaranteeing the safety and effectiveness of healthcare products for public use. Governed by COFEPRIS, these requirements necessitate comprehensive research data from rigorously designed clinical trials that substantiate the manufacturer's performance claims. The classification of devices by risk level—from low-risk Class I to high-risk Class IV—further dictates the extent of documentation required for approval, emphasizing the necessity of understanding these regulations for successful market entry.
Insights into the regulatory framework reveal that adherence to international standards, thorough technical documentation, and effective risk assessments are essential to fulfilling clinical evidence requirements. Manufacturers encounter challenges such as:
These challenges underscore the importance of strategic planning and collaboration with local experts. As the market for medical devices in Mexico expands, these insights become increasingly vital for ensuring compliance and fostering innovation in healthcare technologies.
Addressing the clinical evidence requirements transcends mere regulatory obligation; it represents a commitment to patient safety and confidence in healthcare solutions. Manufacturers are urged to remain informed about evolving regulations and to implement proactive strategies that enhance compliance and streamline the approval process. By prioritizing these efforts, stakeholders can contribute to a robust healthcare system that meets the needs of the Mexican population while upholding the highest standards of safety and efficacy in medical devices.
What organization regulates clinical evidence requirements for medical devices in Mexico?
The clinical evidence requirements for medical devices in Mexico are regulated by COFEPRIS (Federal Commission for Protection against Sanitary Risk).
What type of data must producers provide to meet clinical evidence requirements?
Producers must provide comprehensive research data derived from rigorously designed clinical trials or studies that support the performance claims made by the manufacturer.
How do clinical evidence requirements vary based on device risk classification in Mexico?
The clinical evidence requirements must align with the device's risk classification, which ranges from low-risk Class I to high-risk Class IV. Class I devices typically require less extensive documentation compared to higher-risk categories.
What percentage of healthcare instruments submitted for approval in Mexico are classified as Class I?
Approximately 70% of healthcare instruments submitted for approval in Mexico fall under Class I.
What is the anticipated evaluation period for health equipment registration in 2025?
The anticipated evaluation period for health equipment registration in 2025 is expected to range from 3 to 8 months.
Is there a way to expedite the registration process for medical devices in Mexico?
Yes, there is an option to utilize an Authorized Third Party to expedite registration to as little as 1 to 3 months.
What language must all documentation submitted to COFEPRIS be translated into?
All documentation submitted to COFEPRIS, including foreign clinical study data, must be translated into Spanish.
What is the primary aim of the clinical evidence requirements for medical devices in Mexico?
The primary aim is to ensure that only safe and effective healthcare products are approved for use within the Mexican health system, thereby protecting patient well-being and enhancing confidence in healthcare technologies.