


The article emphasizes the critical role of clinical programming in clinical trials, underscoring its significance in ensuring accurate data management and regulatory compliance. By illustrating its impact on expediting vaccine development and maintaining data integrity, the discussion highlights how clinical programming ultimately contributes to improved patient outcomes and streamlined research processes. This understanding is essential for navigating the complexities of clinical research, making clinical programming a fundamental component of successful trials.
Clinical programming stands at the forefront of medical research, playing a pivotal role in the design and execution of clinical trials. It ensures meticulous management of data and statistical analysis, which not only facilitates regulatory compliance but also enhances the safety and efficacy of new therapies.
However, as the landscape of clinical trials evolves, challenges such as data integrity and compliance persist. This raises a critical question: how can clinical programming adapt to meet these demands while continuing to drive innovation in healthcare?
Clinical programming is a critical procedure in medical research, encompassing the design, execution, and oversight of statistical and information management elements. This involves:
The significance of clinical programming in the development of medical software cannot be overstated; it ensures that information is gathered, examined, and presented with precision, which is vital for regulatory submissions and for making informed decisions regarding the safety and effectiveness of new therapies.
For instance, the rapid advancement of COVID-19 vaccines exemplified how sophisticated statistical techniques could reduce development timelines by 80%, enabling concurrent phase 2 and phase 3 trials. Such efficiency in clinical programming not only accelerates the approval process but also enhances patient outcomes by facilitating quicker access to innovative therapies.
Furthermore, robust research development practices, including clinical programming, are essential for maintaining data integrity, as illustrated by a case where a pharmaceutical company faced a six-month submission delay due to CDISC compliance issues. This highlights the necessity of rigorous quality control procedures in clinical programming for healthcare software development to ensure seamless regulatory submissions.
As Vamsi Upaduri, a senior statistical programmer, remarked, 'SAS laid the foundation as a gold standard with validated procedures, robust audit trails, and comprehensive documentation to ensure regulatory compliance.'
Additionally, with bioaccess's extensive management services for studies, including compliance evaluations and reporting on serious and non-serious adverse events, clinical programming can help achieve 50% faster patient enrollment and significant cost reductions, further underscoring the importance of effective research implementation.
Ultimately, efficient healthcare development, particularly through clinical programming, not only enhances the integrity of research but also significantly contributes to improved patient outcomes and advancements in medical knowledge.

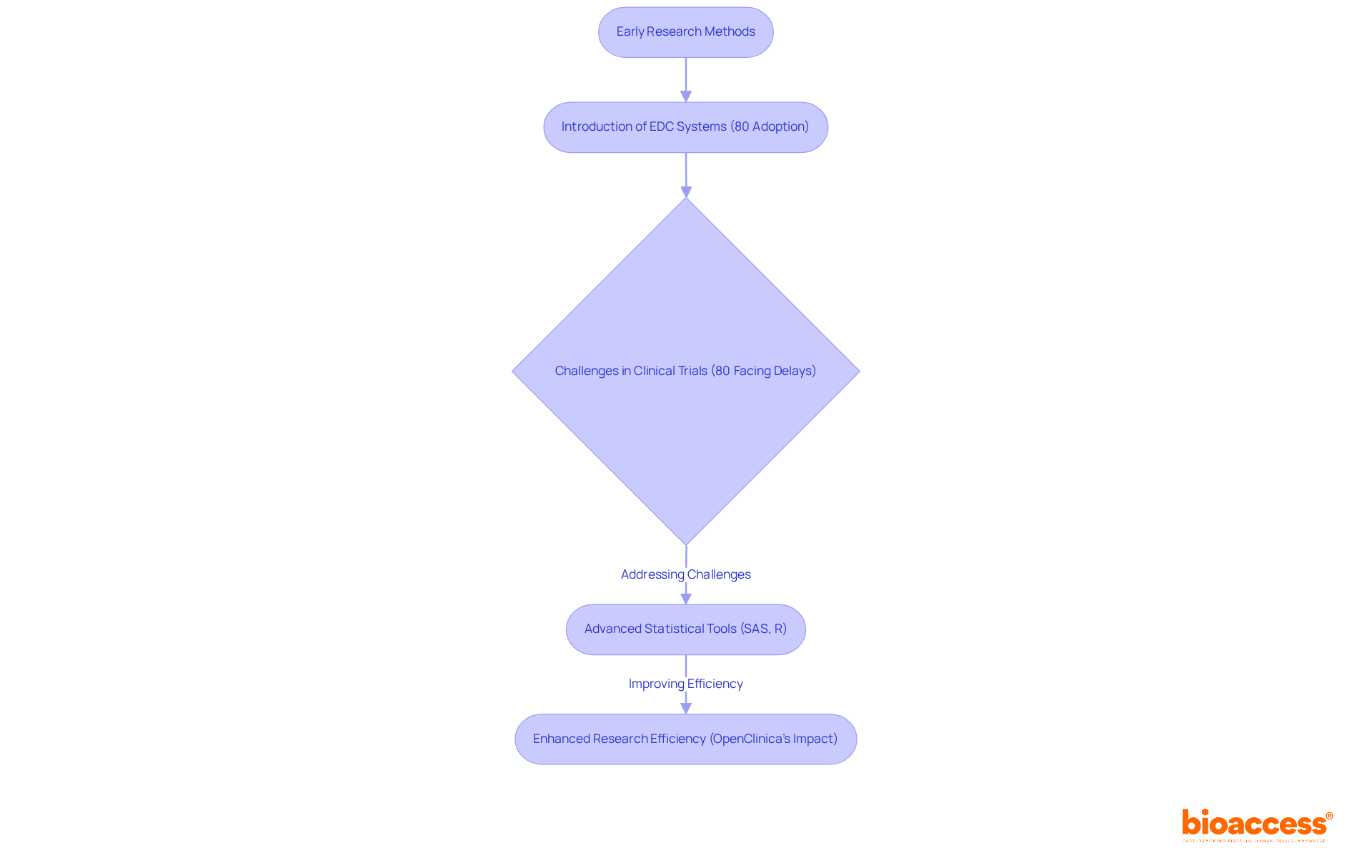
The development of medical programming has undergone significant transformation since the early days of research, which heavily relied on manual and paper-based information gathering techniques. The advent of electronic data capture (EDC) systems represents a pivotal shift, streamlining the processes of data collection and management. Currently, nearly 80% of research studies utilize EDC systems, reflecting a substantial increase in the adoption of digital tools within the industry. Nonetheless, medical trials frequently face delays, with statistics revealing that approximately 80% are impacted, underscoring the challenges that EDC systems aim to address. This transition has been further propelled by the rise of advanced statistical software and coding languages, such as SAS and R, which enable more intricate analyses and enhance overall efficiency.
As clinical programming progresses, it increasingly converges with information science and real-world evidence, aligning with a broader trend towards patient-centered approaches in research. Prominent EDC systems, such as OpenClinica, have garnered recognition for their capacity to significantly minimize integration efforts, requiring less than 20 hours for implementation. Furthermore, OpenClinica's technology can accelerate research studies by as much as 80 percent, illustrating the efficiency gains associated with its application. This evolution not only expedites the research process but also enhances the quality of information management, ultimately leading to more effective and timely outcomes in medical research. As Cal Collins, CEO of OpenClinica, articulates, "It offers a highly reliable method for integrating information across the numerous locations that are typically involved in a research study.
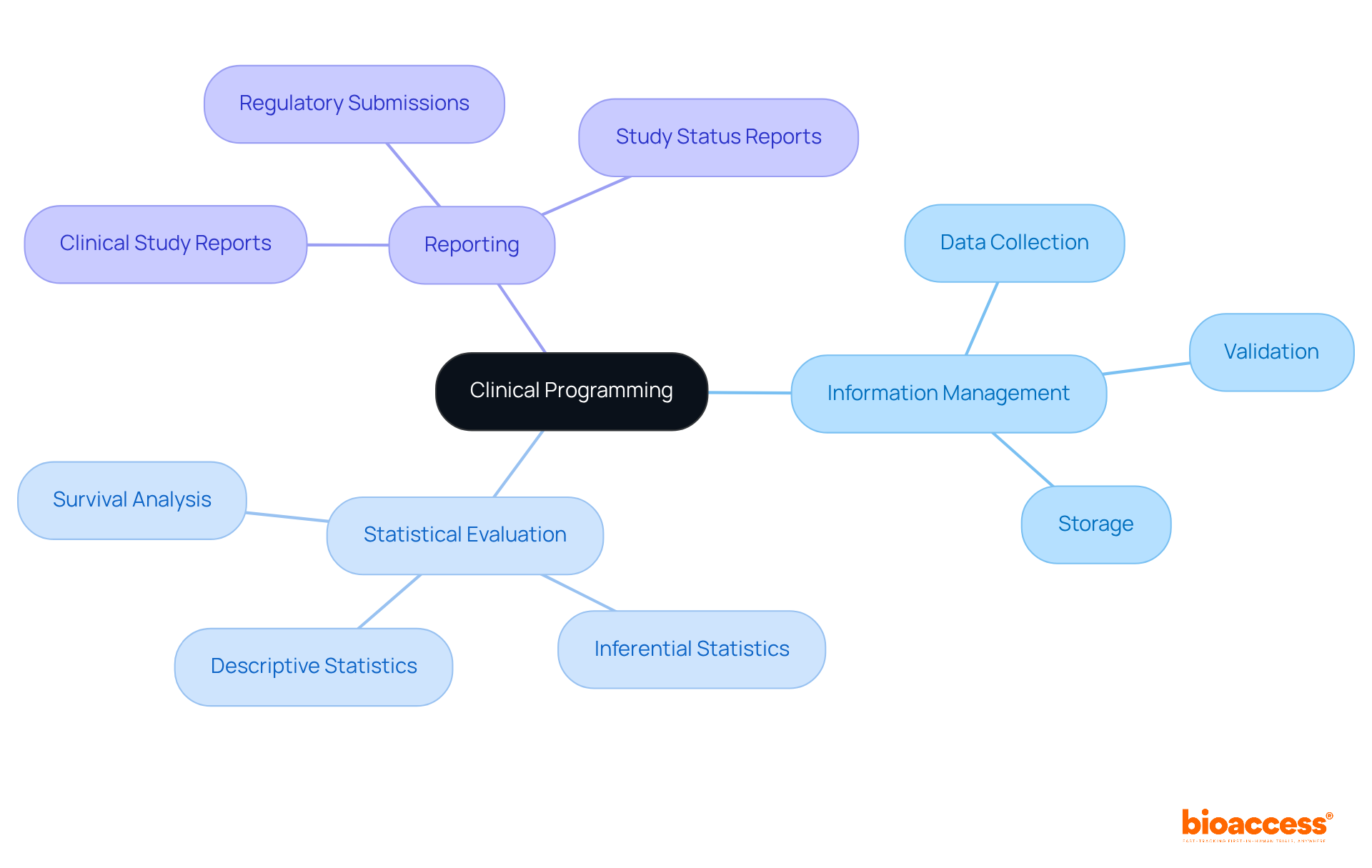
Essential elements of clinical programming encompass information management, statistical evaluation, and reporting, all supported by comprehensive research management services. These services include:
All are vital for the seamless execution of research studies. Information management is pivotal, involving the meticulous collection, validation, and storage of clinical programming data to ensure accuracy and integrity. This process is critical, as high-quality information directly influences the reliability of results. Statistical analysis is instrumental in interpreting this data, employing methodologies such as:
To extract meaningful insights. For example, descriptive statistics summarize participant characteristics and treatment outcomes, while inferential statistics assess the significance of observed effects, with a commonly accepted p-value threshold of 0.05 indicating statistical significance.
Effective communication between statisticians and medical researchers is paramount for successful study design and outcomes, bridging the gap between medical inquiries and statistical methodologies. Reporting involves producing comprehensive documents that summarize the study's findings, including:
Methodologies like Agile and Waterfall are frequently employed in programming to enhance project management, ensuring timely delivery of results while allowing for adjustments to evolving requirements. The integration of robust information management practices and statistical techniques in clinical programming is essential for the success of research studies, as it not only facilitates accurate conclusions but also fosters trust among stakeholders regarding the reliability of the outcomes. Furthermore, the increasing demand for information experts, projected at a growth rate of 36%, underscores the critical importance of efficient information management in the sector. Challenges such as missing data must be addressed, as they can significantly impact the accuracy of research findings. Additionally, the influence of Medtech clinical studies extends beyond the assessments, contributing to local economies through job creation, economic growth, healthcare improvements, and fostering international collaboration.
Clinical programming faces significant challenges, especially concerning information quality, regulatory compliance, and the integration of emerging technologies. The integrity of information is often compromised by human errors and discrepancies in data entry. Research indicates that approximately 25% of entries in Parkinson's disease studies exhibit quality issues, with nearly 46% of entries in Alzheimer's disease studies facing similar concerns.
To mitigate these challenges, it is crucial to implement rigorous validation processes and provide comprehensive training for data entry personnel. Regulatory compliance is paramount; failure to adhere to established guidelines can lead to considerable delays or outright rejection of study results. A national survey revealed that nearly 50% of medical studies did not fully comply with Good Clinical Practice (GCP) guidelines, underscoring the need for improved adherence.
Services offered by bioaccess, such as feasibility studies, site selection, and compliance reviews, can significantly enhance regulatory adherence and streamline research processes. Staying informed about regulatory changes and employing robust documentation practices are essential strategies for ensuring compliance.
Moreover, the rapid advancement of technology demands continuous learning and adaptation. Centralized data monitoring techniques can enable early identification of data issues, while fostering a culture of innovation within clinical programming can enhance the successful integration of new tools and methodologies, ultimately improving the quality and reliability of clinical trial outcomes.

Clinical programming is integral to medical research, serving as the backbone for the design, execution, and oversight of statistical and information management processes in clinical trials. By ensuring meticulous data collection, analysis, and reporting, clinical programming not only supports regulatory compliance but also enhances the safety and efficacy of new therapies. This significance is particularly evident in the rapid advancements observed during the development of COVID-19 vaccines, where clinical programming enabled unprecedented timelines for trial phases.
The evolution of clinical programming is marked by a transition from manual data collection to sophisticated electronic data capture systems, revolutionizing research methodologies. Essential components such as information management, statistical evaluation, and thorough reporting are vital for the integrity and reliability of clinical studies. Moreover, the integration of advanced statistical software and methodologies has bolstered efficiency while addressing challenges related to data quality and regulatory compliance. These insights underscore the necessity for continuous innovation and rigorous quality control to navigate the complexities of modern clinical trials.
In conclusion, the importance of clinical programming is paramount; it drives not only successful clinical trials but also advancements in medical knowledge and improved patient outcomes. As the landscape of clinical research evolves, embracing best practices and addressing emerging challenges will be crucial for the future of healthcare development. Stakeholders in the industry must prioritize the adoption of robust clinical programming methodologies to ensure the continued success and integrity of medical research efforts.
What is clinical programming?
Clinical programming is a critical procedure in medical research that involves the design, execution, and oversight of statistical and information management elements, including creating statistical analysis plans, constructing datasets, and producing detailed reports summarizing study outcomes.
Why is clinical programming important in clinical trials?
Clinical programming is vital for ensuring that information is gathered, analyzed, and presented accurately, which is essential for regulatory submissions and making informed decisions about the safety and effectiveness of new therapies.
How did clinical programming impact the development of COVID-19 vaccines?
Sophisticated statistical techniques in clinical programming allowed for a reduction in development timelines by 80%, enabling concurrent phase 2 and phase 3 trials, which accelerated the approval process and improved patient access to innovative therapies.
What role does clinical programming play in maintaining data integrity?
Robust clinical programming practices are essential for maintaining data integrity, as seen in a case where a pharmaceutical company faced a six-month submission delay due to CDISC compliance issues, emphasizing the need for rigorous quality control procedures.
What is the significance of SAS in clinical programming?
SAS is considered a gold standard in clinical programming due to its validated procedures, robust audit trails, and comprehensive documentation that ensure regulatory compliance.
How can clinical programming improve patient enrollment and reduce costs?
With effective management services for studies, clinical programming can achieve 50% faster patient enrollment and significant cost reductions, highlighting its importance in research implementation.
What overall impact does clinical programming have on healthcare development?
Efficient clinical programming enhances the integrity of research, significantly contributes to improved patient outcomes, and advances medical knowledge.